肌萎缩性侧索硬化症模型中突触功能的实时荧光测量
Summary
描述了两种相关方法,以可视化突触传递所需的亚细胞事件。这些方案能够使用 体外 培养神经元的活细胞成像实时监测突触前钙流入和突触囊泡膜融合的动态。
Abstract
在神经元变性之前,肌萎缩性侧索硬化症(ALS)和/或额颞叶痴呆(FTLD)患者的运动和认知缺陷的原因是神经元与运动神经元和肌肉之间的通信功能障碍。突触传递的潜在过程涉及膜去极化依赖性突触囊泡融合和神经递质释放到突触中。该过程通过局部钙流入突触囊泡所在的突触前末端而发生。在这里,该协议描述了基于荧光的实时成像方法,该方法可靠地报告了培养神经元中去极化介导的突触囊泡胞吐作用和突触前末端钙流入动力学。
使用掺入突触囊泡膜的苯乙烯基染料,阐明突触囊泡释放。另一方面,为了研究钙的进入,使用Gcamp6m,一种遗传编码的荧光报告基因。我们采用高氯化钾介导的去极化来模仿神经元活动。为了明确地量化突触囊泡胞吐作用,我们测量归一化苯乙烯基染料荧光作为时间函数的损失。在类似的刺激条件下,在钙流入的情况下,Gcamp6m荧光增加。这种荧光变化的归一化和定量以与苯乙烯基染料方案类似的方式进行。这些方法可以与基于转染的荧光标记突变蛋白的过表达进行多重检测。这些方案已被广泛用于研究 FUS-ALS和 C9ORF72-ALS模型中的突触功能障碍,利用原代啮齿动物皮质和运动神经元。这些方案很容易允许快速筛选可能改善神经元通讯的化合物。因此,这些方法不仅对ALS的研究有价值,而且对神经退行性和发育性神经科学研究的所有领域都很有价值。
Introduction
由于超过 80% 的病例具有压倒性的散发性,再加上已知是致病性的大量基因突变2,因此在实验室中对肌萎缩性侧索硬化症 (ALS) 进行建模极具挑战性。尽管如此,所有ALS病例都有一个统一的特征,即在完全神经元变性之前,突触前运动神经元和突触后肌肉细胞之间存在功能障碍的通信3,4。临床上,当患者失去剩余的上下运动神经元的连接时,它们在整个疾病过程中表现出神经元过度和低兴奋性的特征5,6,7,8,9,反映了这些突触的复杂潜在分子变化,我们作为ALS研究人员试图理解这一点。
多种转基因模型表明,神经肌肉接头的恶化和紊乱随着ALS致病基因突变的表达而发生,包括SOD110,FUS11,12,C9orf7213,14,15,16和TDP4317,18,19 通过形态学评估,包括突触胸顿、脊柱密度和突触前/突触后组织的评估。从机制上讲,自20世纪30年代科尔,霍奇金和赫胥黎的里程碑式论文以来,还可以通过体外细胞培养或组织切片制剂中的电生理技术评估突触反应20。通过这些策略,许多ALS模型已经显示出突触传递缺陷。例如,TDP43的突变变体导致NSC-34(脊髓x神经母细胞瘤杂交细胞系34)运动神经元样细胞中的放电频率增强并降低动作电位阈值21。在小鼠模型中,这种相同的变异还会导致神经肌肉接头(NMJ)处的功能失调性突触传递,然后才出现行为运动缺陷22。先前显示,在运动缺陷发生之前,FUS-ALS果蝇模型中的突变FUS表达导致NMJ突触传递减少11。最近一份使用来自C9orf72扩增载体的诱导多能干细胞的报告显示,易释放的突触囊泡池减少23。总而言之,这些研究和其他研究强调了对ALS疾病相关模型中突触信号传导的潜在机制建立更全面理解的重要性。这对于了解ALS的病理生物学和为患者开发潜在的治疗靶点至关重要。
电流和电压钳位单元的方法在确定膜性质方面具有无可估量的价值,例如电导率,静息膜电位和单个突触的量子含量20,24。然而,电生理学的一个重大局限性是,它在技术上具有挑战性,并且一次只能提供来自单个神经元的见解。活细胞共聚焦显微镜,加上特定的荧光探针,为以时空方式研究神经元的突触传递提供了机会25,26,27。虽然不是神经元兴奋性的直接测量,但这种荧光方法可以相对测量突触功能的两种分子相关性:突触囊泡释放和突触末端的钙瞬变。
当动作电位到达神经元的突触前末端区域时,钙瞬变被触发,促进从电信号到神经递质释放过程的转变28。定位于这些区域的电压门控钙通道严格调节钙信号传导,以调节神经递质释放的动力学29。首次报道的基于荧光的钙瞬变记录是使用双波长指示剂Fura-2 AM或单波长染料Flu-3 AM30,31,32进行的。虽然这些染料在当时提供了很好的新见解,但它们存在一些局限性,例如细胞内非特异性区室化,标记细胞的主动或被动染料损失,光漂白以及长时间成像的毒性33。在过去的十年中,遗传编码的钙指示剂已成为对各种形式的神经元活动进行成像的主力军。这些指示剂将修饰的荧光蛋白与钙螯合蛋白结合,钙螯合蛋白在Ca2 + 离子结合后快速切换荧光强度34。这些新指标的应用范围很广,可以在体外和体内环境中更容易地可视化细胞内钙瞬变。这些基因编码报告基因的一个家族,称为GCaMP,现在被广泛使用。这些指示剂包含C端钙调蛋白结构域,后跟绿色荧光蛋白(GFP),并被N端钙调蛋白结合区覆盖35,36。钙与钙调蛋白结构域的结合触发与钙调蛋白结合区域的相互作用,导致整体蛋白质结构的构象变化和GFP部分荧光的显着增加35,36。多年来,这个报告员家族经历了几次演变,以便为具有特定动力学(慢,中和快)的特定钙瞬变提供不同的读数,每个都具有略微不同的性质37,38。在这里,已经强调了报告者GcaMP6的使用,其先前已被证明可以在体内和体外检测神经元中的单次动作电位和树突状钙瞬变37。
突触前区域的钙瞬变触发突触囊泡融合事件,导致神经递质释放到突触中并在突触后细胞中启动信号传导事件28,39。突触囊泡既能快速释放又可循环,因为细胞稳态地维持稳定的细胞膜表面积和易于释放的融合池,具有膜结合的囊泡40。这里使用的苯乙烯基染料对脂质膜具有亲和力,并根据周围脂质环境的顺序特异性地改变其发射特性41,42。因此,它是标记回收突触囊泡并随后跟踪这些囊泡的理想工具,因为它们随后在神经元刺激后释放41,42。已经生成和优化的方案是对Gaffield及其同事最初描述的概念的改编,这使我们能够随着时间的推移连续地可视化苯乙烯染料标记的突触囊泡点41。
在这里,描述了两种相关的基于荧光的方法,可靠地报告了突触传递中涉及的特定细胞事件。已经定义了方案来探测培养神经元中去极化介导的突触前终末期钙流入和突触囊泡胞吐作用的动力学。在这里,方法和代表性结果侧重于使用原代啮齿动物皮质或运动神经元作为体外模型系统,因为有已发表的使用这些细胞类型的研究43,44。然而,这些方法也适用于分化的人类i3皮质样神经元45,因为我们在实验室目前正在进行的实验中也成功地使用了这两种方案。一般协议以逐步线性格式概述,如图1所示。简而言之,为了研究神经突起中的钙动力学,成熟的神经元被质粒DNA转染,以在巨细胞病毒(CMV)启动子下表达荧光报告GCaMP6m37,46。转染的细胞具有低水平的基底绿色荧光,在钙的存在下增加。指定感兴趣的区域以监测整个操作过程中的荧光变化。这允许测量钙的高度空间和时间局部波动37,46。为了评估突触囊泡融合和释放,成熟的神经元被加载到掺入突触囊泡膜中的苯乙烯染料,因为它们被回收,重整并重新加载突触前细胞中的神经递质41,42,43,47,48。目前用于此目的的染料沿着神经突触囊泡标记,并在实时成像实验中用作这些区域的代表,正如Kraszewski及其同事对苯乙烯染料和突触标记素的共染色所显示的那样49。这里包括也进行了类似染色的代表性图像(图2A)。以前的研究人员广泛使用这种染料来报告神经肌肉接头和海马神经元的突触囊泡动力学48,49,50,51,52,53,54,55,56.通过选择含染料囊泡的点状区域并监测囊泡释放后荧光强度的降低,可以研究刺激后的功能突触传递能力和释放的时间动力学43。对于这两种方法,使用含有高浓度氯化钾的培养基来去极化细胞以模仿神经元活动。指定成像参数以捕获跨越基线归一化和刺激捕获周期的亚秒间隔。确定每个时间点的荧光测量值,归一化到背景,并在实验时间段内进行量化。钙流入介导的GCaMP6m荧光增加或有效的突触囊泡胞吐作用苯乙烯染料释放荧光减少可以通过该策略检测到。下面描述了这两种方案的详细方法设置和参数,并讨论了它们的优点和局限性。
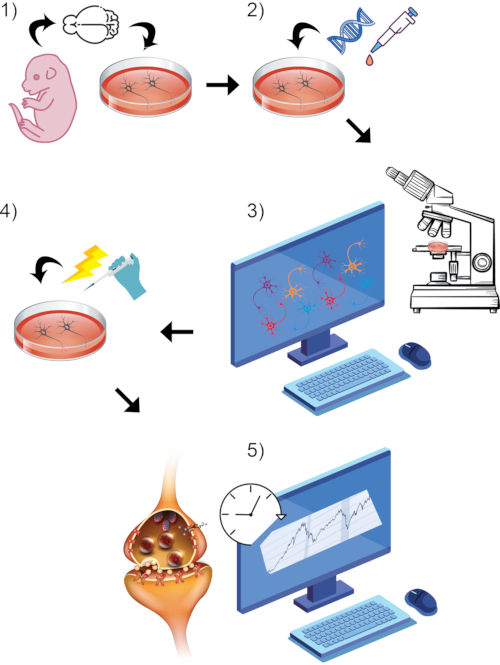
图 1:整体通用协议流程的可视化呈现。 (1) 在体外 分离并培养原代啮齿动物神经元到选定的成熟时间点。(2)引入GCaMP DNA或苯乙烯基染料作为突触活性的报告者。(3)使用配备实时成像的共聚焦显微镜和相关软件设置成像范例。开始基线记录期。(4)当细胞仍在进行实时图像捕获时,通过高KCl浴灌注刺激神经元。(5)评估荧光强度随时间的变化,以测量钙瞬变或突触囊泡融合。 请点击此处查看此图的放大版本。
Protocol
Representative Results
Discussion
所描述的两种方法共有的三个步骤对于实验成功和可量化的结果至关重要。首先,按照所附说明,在每轮实验之前准备新鲜的aCSF是必不可少的。如果不这样做,可能会阻止适当的神经元去极化。在刺激任何实验组之前,应不断测试未经治疗的对照神经元样本,以确保适当的细胞去极化,并为在该成像过程中获得的积极结果提供基准。其次,为了成功跟踪特定突触区域随时间变化的荧光,在设置成…
Disclosures
The authors have nothing to disclose.
Acknowledgements
我们要感谢杰斐逊·温伯格ALS中心的现任和前任成员,他们为优化这些技术及其分析提供了重要的反馈和建议。这项工作得到了NIH(RF1-AG057882-01和R21-NS0103118到D.T),NINDS(R56-NS092572和R01-NS109150到P.P),肌肉萎缩症协会(D.T.),罗伯特帕卡德ALS研究中心(D.T.),Family Strong 4 ALS基金会和Farber家庭基金会(B.K.J.,K.K.和P.P.)的资助。
Materials
| 20x air objective | Nikon | For imaging | |
| 40x oil immersion objective | Nikon | For imaging | |
| B27 supplement | Thermo Scientific | 17504044 | Neuronal growth supplement |
| BD Syringes without Needle, 50 mL | Thermo Scientific | 13-689-8 | Part of gravity perfusion assembly |
| Biosafety cell culture hood | Baker | SterilGARD III SG403A | Asceptic cell culturing, transfection, and dye loading |
| b-Mercaptoethanol | Millipore Sigma | M3148 | For culturing and maintenance of neuronal cultures |
| Bovine Serum Albumin | Millipore Sigma | A9418 | For preparing neuronal cultures |
| Calcium chloride dihydrate | Millipore Sigma | 223506 | Component of aCSF solutions |
| Cell culture CO2 incubator | Thermo Scientific | 13-998-123 | For culturing and maintenance of neurons |
| Centrifuge | Eppendorf | 5810R | For neuronal culture preparation |
| Confocal microscope | Nikon | Eclipse Ti +A1R core | For fluorescence imaging |
| CoolSNAP ES2 CCD camera | Photometrics | For image acquisition | |
| D-Glucose | Millipore Sigma | G8270 | Component of aCSF solutions |
| DNase | Millipore Sigma | D5025 | For neuronal culture preparation |
| Female, timed-pregnancy Sprague Dawley rats | Charles river | 400SASSD | For preparing embryonic cortical and spinal motor neuron cultures |
| FITC Filter cube | Nikon | 77032509 | For imaging Gcamp calcium transients |
| FM4-64 styryl dye | Invitrogen | T13320 | For imaging synaptic vesicle release |
| Glass bottom petri dishes (Thickness #1.5) | CellVis | D35-10-1.5-N | For growth of neurons on imaging-compatible culture dish |
| Glass Pasteur pipette | Grainger | 52NK56 | For preparing neuronal cultures |
| Hank's Balanced Salt Solution (HBSS) | Millipore Sigma | H6648 | For preparing neuronal cultures |
| HEPES | Millipore Sigma | H3375 | Component of aCSF solutions |
| High KCl artifical cerebrospinal fluid (aCSF) | For imaging. Please see recipes* | ||
| horse serum | Millipore Sigma | H1138 | For culturing and maintenance of neurons |
| Laminar flow dissection hood | NUAIRE | NU-301-630 | For preparing neuronal cultures |
| Laminin | Thermo Scientific | 23017015 | For preparing neuronal cultures |
| Leibovitz's L-15 Medium | Thermo Scientific | 11415064 | For preparing neuronal cultures |
| Leibovitz's L-15 Medium, no phenol red | Thermo Scientific | 21083027 | For preparing neuronal cultures |
| L-Glutamine (200 mM) | Thermo Scientific | 25030149 | Neuronal culture supplement |
| Lipofectamine 2000 Transfection Reagent | Thermo Scientific | 11668019 | For neuronal transfections |
| Low KCl artifical cerebrospinal fluid (aCSF) | For imaging. Please see recipes* | ||
| Magnesium chloride | Millipore Sigma | 208337 | Component of aCSF solutions |
| Microsoft Excel | Microsoft | Software for data analysis/normalization | |
| Nalgene Filter Units, 0.2 µm PES | Thermo Scientific | 565-0020 | Filter unit for aCSF solution |
| Neurobasal medium | Thermo Scientific | 21103049 | For culturing and maintenance of neuronal cultures |
| NIS-Elements Advanced Research | Nikon | Software for image capture and analysis | |
| Nunc 15 mL Conical tubes | Thermo Scientific | 339650 | For preparing neuronal culture and buffer solutions |
| Nunc 50 mL conical tubes | Thermo Scientific | 339652 | For preparing neuronal culture and buffer solutions |
| Optiprep | Millipore Sigma | D1556 | For preparing neuronal cultures |
| Papain | Millipore Sigma | P4762 | For preparing neuronal cultures |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Scientific | 15140122 | To prevent bacterial contamination of neuronal cultures |
| Perfusion system | Warner Instruments | SF-77B | For exchange of aCSF |
| Perfusion tubing | Cole-Parmer | UX-30526-14 | Part of gravity perfusion assembly |
| pGP-CMV-Gcamp6m plasmid | Addgene | 40754 | For imaging calcium transients |
| Poly-D-lysine hydrobromide | Millipore Sigma | P7886 | Coating agent for glass bottom petri dishes |
| Potassium chloride | Millipore Sigma | P3911 | Component of aCSF solutions |
| Sodium bicarbonate | Millipore Sigma | S5761 | Component of aCSF solutions |
| Sodium Chloride | Millipore Sigma | S9888 | Component of aCSF solutions |
| Stage Top Incubator | Tokai Hit | For incubation of live neurons during imaging period | |
| TRITC Filter cube | Nikon | 77032809 | For imaging FM4-64 |
| Trypsin Inhibitor | Millipore Sigma | T6414 | For preparing neuronal cultures |
| Trypsin-EDTA (0.25%), phenol red | Thermo Scientific | 25200056 | For preparing neuronal cultures |
| Vibration Isolation table | New Port | VIP320X2430-135520 | Table/stand for microscope |
References
- Gibson, S. B., et al. The evolving genetic risk for sporadic ALS. Neurology. 89 (3), 226-233 (2017).
- Kim, G., Gautier, O., Tassoni-Tsuchida, E., Ma, X. R., Gitler, A. D. ALS genetics: Gains, losses, and implications for future therapies. Neuron. 108 (5), 822-842 (2020).
- Nijssen, J., Comley, L. H., Hedlund, E. Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis. Acta Neuropathologica. 133 (6), 863-885 (2017).
- Marttinen, M., Kurkinen, K. M., Soininen, H., Haapasalo, A., Hiltunen, M. Synaptic dysfunction and septin protein family members in neurodegenerative diseases. Molecular Neurodegeneration. 10, 16 (2015).
- Bae, J. S., Simon, N. G., Menon, P., Vucic, S., Kiernan, M. C. The puzzling case of hyperexcitability in amyotrophic lateral sclerosis. Journal of Clinical Neurology. 9 (2), 65-74 (2013).
- Kiernan, M. C. Hyperexcitability, persistent Na+ conductances and neurodegeneration in amyotrophic lateral sclerosis. Experimental Neurology. 218 (1), 1-4 (2009).
- Krarup, C. Lower motor neuron involvement examined by quantitative electromyography in amyotrophic lateral sclerosis. Clinical Neurophysiology. 122 (2), 414-422 (2011).
- Vucic, S., Nicholson, G. A., Kiernan, M. C. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 131, 1540-1550 (2008).
- Marchand-Pauvert, V., et al. Absence of hyperexcitability of spinal motoneurons in patients with amyotrophic lateral sclerosis. Journal of Physiology. 597 (22), 5445-5467 (2019).
- Fischer, L. R., et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Experimental Neurology. 185 (2), 232-240 (2004).
- Markert, S. M., et al. Overexpression of an ALS-associated FUS mutation in C. elegans disrupts NMJ morphology and leads to defective neuromuscular transmission. Biology Open. 9 (12), (2020).
- Shahidullah, M., et al. Defects in synapse structure and function precede motor neuron degeneration in Drosophila models of FUS-related ALS. Journal of Neuroscience. 33 (50), 19590-19598 (2013).
- Liu, Y., et al. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron. 90 (3), 521-534 (2016).
- Freibaum, B. D., et al. GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature. 525 (7567), 129-133 (2015).
- Zhang, K., et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 525 (7567), 56-61 (2015).
- Perry, S., Han, Y., Das, A., Dickman, D. Homeostatic plasticity can be induced and expressed to restore synaptic strength at neuromuscular junctions undergoing ALS-related degeneration. Human Molecular Genetics. 26 (21), 4153-4167 (2017).
- Romano, G., et al. Chronological requirements of TDP-43 function in synaptic organization and locomotive control. Neurobiology of Disease. 71, 95-109 (2014).
- Armstrong, G. A., Drapeau, P. Calcium channel agonists protect against neuromuscular dysfunction in a genetic model of TDP-43 mutation in ALS. Journal of Neuroscience. 33 (4), 1741-1752 (2013).
- Diaper, D. C., et al. Loss and gain of Drosophila TDP-43 impair synaptic efficacy and motor control leading to age-related neurodegeneration by loss-of-function phenotypes. Human Molecular Genetics. 22 (8), 1539-1557 (2013).
- Schwiening, C. J. A brief historical perspective: Hodgkin and Huxley. Journal of Physiology. 590 (11), 2571-2575 (2012).
- Dong, H., et al. Curcumin abolishes mutant TDP-43 induced excitability in a motoneuron-like cellular model of ALS. Neuroscience. 272, 141-153 (2014).
- Chand, K. K., et al. Defects in synaptic transmission at the neuromuscular junction precede motor deficits in a TDP-43(Q331K) transgenic mouse model of amyotrophic lateral sclerosis. Federation of American Societies for Experimental Biology Journal. 32 (5), 2676-2689 (2018).
- Perkins, E. M., et al. Altered network properties in C9ORF72 repeat expansion cortical neurons are due to synaptic dysfunction. Molecular Neurodegeneration. 16 (1), 13 (2021).
- Ceccarelli, B., Hurlbut, W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiological Reviews. 60 (2), 396-441 (1980).
- Ettinger, A., Wittmann, T. Fluorescence live cell imaging. Methods in Cell Biology. 123, 77-94 (2014).
- Ryan, J., Gerhold, A. R., Boudreau, V., Smith, L., Maddox, P. S. Introduction to Modern Methods in Light Microscopy. Methods in Molecular Biology. 1563, 1-15 (2017).
- Wang, L., Frei, M. S., Salim, A., Johnsson, K. Small-molecule fluorescent probes for live-cell super-resolution microscopy. Journal of the American Chemical Society. 141 (7), 2770-2781 (2019).
- Neher, E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 20 (3), 389-399 (1998).
- Dolphin, A. C., Lee, A. Presynaptic calcium channels: specialized control of synaptic neurotransmitter release. Nature Reviews Neuroscience. 21 (4), 213-229 (2020).
- Tsien, R. Y., Rink, T. J., Poenie, M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 6 (1-2), 145-157 (1985).
- Takahashi, N., et al. Cytosolic Ca2+ dynamics in hamster ascending thin limb of Henle’s loop. American Journal of Physiology. 268 (6), 1148-1153 (1995).
- Cleemann, L., DiMassa, G., Morad, M. Ca2+ sparks within 200 nm of the sarcolemma of rat ventricular cells: evidence from total internal reflection fluorescence microscopy. Advances in Experimental Medicine and Biology. 430, 57-65 (1997).
- Roe, M. W., Lemasters, J. J., Herman, B. Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium. 11 (2-3), 63-73 (1990).
- Lin, M. Z., Schnitzer, M. J. Genetically encoded indicators of neuronal activity. Nature Neuroscience. 19 (9), 1142-1153 (2016).
- Tian, L., et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 6 (12), 875-881 (2009).
- Nakai, J., Ohkura, M., Imoto, K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nature Biotechnology. 19 (2), 137-141 (2001).
- Chen, T. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Horikawa, K. Recent progress in the development of genetically encoded Ca2+ indicators. Journal of Medical Investigation. 62 (1-2), 24-28 (2015).
- Bohme, M. A., Grasskamp, A. T., Walter, A. M. Regulation of synaptic release-site Ca(2+) channel coupling as a mechanism to control release probability and short-term plasticity. Federation of European Biochemical Society Letters. 592 (21), 3516-3531 (2018).
- Li, Y. C., Kavalali, E. T. Synaptic vesicle-recycling machinery components as potential therapeutic targets. Pharmacological Reviews. 69 (2), 141-160 (2017).
- Gaffield, M. A., Betz, W. J. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nature Protocols. 1 (6), 2916-2921 (2006).
- Verstreken, P., Ohyama, T., Bellen, H. J. FM 1-43 labeling of synaptic vesicle pools at the Drosophila neuromuscular junction. Methods in Molecular Biology. 440, 349-369 (2008).
- Jensen, B. K., et al. Synaptic dysfunction induced by glycine-alanine dipeptides in C9orf72-ALS/FTD is rescued by SV2 replenishment. European Molecular Biology Organization Molecular Medicine. 12 (5), 10722 (2020).
- Kia, A., McAvoy, K., Krishnamurthy, K., Trotti, D., Pasinelli, P. Astrocytes expressing ALS-linked mutant FUS induce motor neuron death through release of tumor necrosis factor-alpha. Glia. 66 (5), 1016-1033 (2018).
- Fernandopulle, M. S., et al. Transcription Factor-Mediated Differentiation of Human iPSCs into Neurons. Current Protocols in Cell Biology. 79 (1), 51 (2018).
- Ye, L., Haroon, M. A., Salinas, A., Paukert, M. Comparison of GCaMP3 and GCaMP6f for studying astrocyte Ca2+ dynamics in the awake mouse brain. Public Library of Science One. 12 (7), 0181113 (2017).
- Angleson, J. K., Betz, W. J. Monitoring secretion in real time: capacitance, amperometry and fluorescence compared. Trends in Neuroscience. 20 (7), 281-287 (1997).
- Ryan, T. A., et al. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 11 (4), 713-724 (1993).
- Kraszewski, K., et al. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. Journal of Neuroscience. 15 (6), 4328-4342 (1995).
- Betz, W. J., Mao, F., Bewick, G. S. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. Journal of Neuroscience. 12 (2), 363-375 (1992).
- Betz, W. J., Bewick, G. S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 255 (5041), 200-203 (1992).
- Ryan, T. A., Smith, S. J. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 14 (5), 983-989 (1995).
- Betz, W. J., Ridge, R. M., Bewick, G. S. Comparison of FM1-43 staining patterns and electrophysiological measures of transmitter release at the frog neuromuscular junction. Journal of Physiology-Paris. 87 (3), 193-202 (1993).
- Wu, L. G., Betz, W. J. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 17 (4), 769-779 (1996).
- Ryan, T. A., Smith, S. J., Reuter, H. The timing of synaptic vesicle endocytosis. Proceedings of the National Academy of Sciences of the United States of America. 93 (11), 5567-5571 (1996).
- Ramaswami, M., Krishnan, K. S., Kelly, R. B. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 13 (2), 363-375 (1994).
- Kayser, M. S., McClelland, A. C., Hughes, E. G., Dalva, M. B. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. Journal of Neuroscience. 26 (47), 12152-12164 (2006).
- Washburn, H. R., Xia, N. L., Zhou, W., Mao, Y. T., Dalva, M. B. Positive surface charge of GluN1 N-terminus mediates the direct interaction with EphB2 and NMDAR mobility. Nature Communications. 11 (1), 570 (2020).
- Magrane, J., Sahawneh, M. A., Przedborski, S., Estevez, A. G., Manfredi, G. Mitochondrial dynamics and bioenergetic dysfunction is associated with synaptic alterations in mutant SOD1 motor neurons. Journal of Neuroscience. 32 (1), 229-242 (2012).
- Casci, I., et al. Muscleblind acts as a modifier of FUS toxicity by modulating stress granule dynamics and SMN localization. Nature Communications. 10 (1), 5583 (2019).
- Hruska, M., Henderson, N., Le Marchand, S. J., Jafri, H., Dalva, M. B. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nature Neuroscience. 21 (5), 671-682 (2018).
- Rein, M. L., Deussing, J. M. The optogenetic (r)evolution. Molecular Genetics and Genomics. 287 (2), 95-109 (2012).
- Bertucci, C., Koppes, R., Dumont, C., Koppes, A. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Research Bulletin. 152, 265-284 (2019).
- Zhang, Y., et al. jGCaMP8 Fast genetically encoded calcium indicators. Janelia Research Campus. , (2020).
- Guerra-Gomes, S., Sousa, N., Pinto, L., Oliveira, J. F. Functional roles of astrocyte calcium elevations: From synapses to behavior. Frontiers in Cellular Neuroscience. 11, 427 (2017).
- Westergard, T., et al. Cell-to-cell transmission of dipeptide repeat proteins linked to C9orf72-ALS/FTD. Cell Reports. 17 (3), 645-652 (2016).
- Wen, X., et al. Antisense proline-arginine RAN dipeptides linked to C9ORF72-ALS/FTD form toxic nuclear aggregates that initiate in vitro and in vivo neuronal death. Neuron. 84 (6), 1213-1225 (2014).
- Daigle, J. G., et al. Pur-alpha regulates cytoplasmic stress granule dynamics and ameliorates FUS toxicity. Acta Neuropathologica. 131 (4), 605-620 (2016).

