用于硅平面皮质内微电极表面处理的工具
Summary
本方案描述了在处理过程中处理硅平面皮质内微电极的工具, 以便通过 气体沉积和水溶液反应进行表面改性。详细解释了在整个过程中用于处理设备的组件的组装。
Abstract
皮质内微电极具有巨大的治疗潜力。但是,他们面临的挑战是在适度的植入时间后显着降低性能。观察到的下降的一个重要因素是植入物近端神经组织的损伤和随后的神经炎症反应。提高设备寿命的努力包括化学修饰或将应用涂覆到设备表面以改善组织响应。这种表面处理的开发通常使用非功能性“假”探头完成,这些探头缺乏预期应用所需的电气元件。鉴于皮质内微电极阵列的脆弱性,转换为功能器件需要额外的考虑。处理工具极大地促进了对组装设备的表面处理,特别是对于需要较长程序时间的修改。此处描述的处理工具用于 通过 气相沉积和水溶液暴露进行的表面处理。涂层的表征使用椭圆偏振和X射线光电子能谱进行。在功能器件上涂覆过程之前和之后的电阻抗谱记录的比较确认了修改后的器件完整性。所描述的工具可以很容易地适应于保持化学相容性的替代电极设备和治疗方法。
Introduction
神经假体装置旨在恢复各种患者群体中受损或缺失的感觉和运动能力,包括脊髓损伤、肌萎缩性侧索硬化症 (ALS)、脑瘫和截肢1,2,3。皮质内微电极(IME)可以在皮质神经元和用于控制神经假体的设备之间建立通信途径。皮质内微电极的一个明显优点是它们能够以高空间和时间分辨率记录神经信号,这是随后的信号处理和控制脑机接口4,5的首选。不幸的是,皮质内微电极的性能在植入后的几个月内急剧下降到一年2,6,7,8。信号质量和稳定性的损失会对该技术的应用产生负面影响。
观察到的性能下降的一个重要因素是对植入相关组织损伤和慢性神经炎症的生物反应9,10,11。IME的植入会对脑组织造成损害,导致信号分子的释放,从而引发反应性细胞防御过程的级联。慢性接口会加剧异物反应,导致持续的神经炎症,损害设备近端的组织;通常被认为是神经炎症,瘢痕形成和局部神经变性的症状,导致信号质量记录下降12,13,14,15。包括具有夹带活化小胶质细胞和巨噬细胞的致密星形胶质细胞团,封装电极的疤痕创造了不利的局部环境,物质转运减少和炎症因子的局部积累16,15,16,17,18。
许多研究描述了大脑对皮质内微电极的反应或减轻反应的方法7。改善组织反应的研究和开发涉及一系列策略,包括对整体结构,表面拓扑,材料和涂层应用的修改。这些努力旨在最大限度地减少植入事件造成的损害,在装置和近端细胞之间引入更有利的界面,或在装置植入7后减少组织应变。专门针对慢性生物反应的方法已经产生了几种生物活性涂层,旨在稳定着床部位并化学促进细胞健康。实例包括导电聚合物如聚(乙烯-二氧噻吩)(PEDOT)19,20、碳纳米管21、水凝胶22,以及添加生物活性分子和药物以靶向特定的细胞过程23、24、25。特别是我们的研究小组,已经探索了许多促进对植入微电极的炎症反应减少的机制,包括但不限于最小化与设备植入相关的创伤26,最小化设备/组织刚度不匹配27,28,29,30,31,32,33,优化灭菌程序34,35,还原氧化应激/损伤28,36,37,38,39,40,41,42,探索替代电极材料43,并模仿天然细胞外基质的纳米结构44,45,46.最近的兴趣是仿生表面涂层的发展,以减轻微电极组织界面39处的神经炎症反应。
界面的修改提供了直接靶向伤口和信号记录所需的近端组织的独特好处。在不加剧免疫反应的情况下促进愈合的表面处理可以有益于质量记录的寿命,并消除实现皮质内微电极的治疗和研究潜力的限制。所介绍的工作详细介绍了将表面处理应用于微电极阵列的方法,这些方法需要延长反应时间,同时适应器件的脆弱性。所提出的技术旨在将表面改性方法共享给功能设备,其中设备在整个处理应用中无法处理。这些工具用于处理非功能性假探头和功能性硅平面微电极阵列。
所提出的修改电极表面的方法允许非功能性假探针或功能性硅平面电极阵列的安全悬浮,用于气相沉积和与水溶液的反应。几个3D打印件用于处理这些易碎的设备(图1 和 图2)。提供了一个程序示例,该程序利用气体和溶液相步骤进行表面改性,使用涉及Mn(III)四(4-苯甲酸)卟啉(MnTBAP)固定化的抗氧化涂层。MnTBAP是一种合成的金属卟啉,具有抗氧化特性,并已证明可以调解炎症47,48。提供的关于功能性硅平面电极阵列的示例验证了对先前报告的用于非功能性设备40的协议的更新。Munief等人 对气相沉积技术的适应性支持该协议与功能电极49的兼容性。气相沉积用于胺官能化表面,为涉及碳二亚胺交联剂化学的水反应做准备,以固定活性MnTBAP。这里开发的处理方法作为一个平台提供,可以对其进行修改以适应其他涂层和类似设备。
该协议说明了使用非功能性假探头的方法,这些探头包括硅柄和3D打印标签,其尺寸与功能性硅平面电极阵列相似。该设备的连接器包装被认为类似于所提供的说明中非功能性假探头的3D打印选项卡。
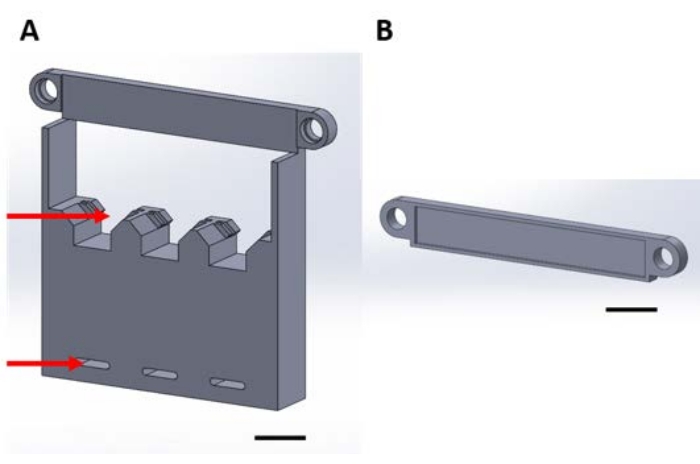
图 1:用于在真空干燥器中气相沉积期间处理功能器件的 3D 打印件 (A) 结构的底座包括用于 1 cm x 1 cm 样品硅方块的支架(顶部箭头)和用于固定到干燥器板的孔(底部箭头)。(B)板用于固定设备的悬挂。从这里开始,此图中的每个部分将称为部分1A或1B。比例尺 = 1 cm .请点击此处查看此图的放大图。
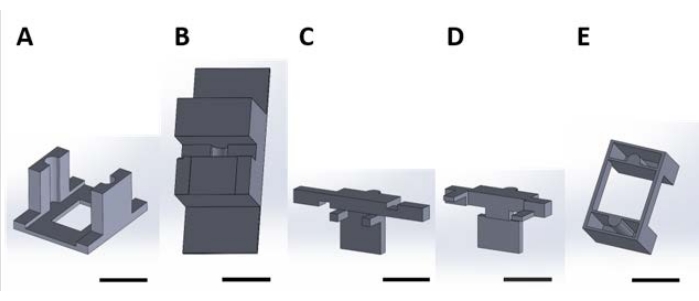
图2:用于处理水溶液中发生表面反应的功能器件的3D打印件。(B) 用于在组装时稳定 (C) 和 (D) 件的台式件。(C)和(D)一起固定装置的悬浮液以放置在孔板中,并且(E)进一步将(C)和(D)片固定在孔板盖上。从这里开始,此图的每个面板中的单个部件将称为与该图的面板编号相对应的部件号。比例尺 = 1 cm.请点击此处查看此图的放大图。
Protocol
Representative Results
Discussion
所述方案设计用于硅平面微电极阵列的表面处理。3D打印工具定制为密歇根风格的微电极阵列,带有薄型连接器50。非功能性探针是通过使用生物相容性粘合剂将硅探针粘附到3D打印标签上来组装的。3D打印的标签设计与所使用的商用设备上的连接器具有相似的尺寸。3D 打印选项卡的文件可用作 补充编码文件 15、补充编码文件 16。丙烯腈丁二烯苯乙烯(ABS)长丝用于?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
这项研究得到了美国退伍军人事务部康复研究与发展服务部的优异审查奖IRX002611(卡帕多纳)和研究职业科学家奖IK6RX003077(卡帕多纳)的部分支持。此外,这项工作还得到了美国国立卫生研究院,国家神经疾病和中风研究所R01NS110823(Capadona / Pancrazio)以及美国国家科学基金会研究生研究奖学金计划(克雷布斯)的部分支持。
Materials
| 1-[3-(Dimethylamino)propyl]-3-ethylcarbodiimide methiodide (EDC) | Sigma-Aldrich | 165344-1G | Solid, stored desiccated at -20 °C |
| 15 mL Conical Centrifuge Tubes | Fisher Scientific | 14-959-70C | |
| 18 Pound Solid Nylon Cable/Zip Ties | Cole-Parmer | EW-06830-66 | Length 4 inches |
| 2-(N-Morpholino)ethanesulfonic acid (MES) | Sigma-Aldrich | 4432-31-9 | Solid |
| 3-aminopropyltriethoxysilane (APTES) | Sigma-Aldrich | 440140-100ML | Liquid, container with Sure/Seal |
| 50 mL Conical Centrifuge Tubes | Fisher Scientific | 14-959-49A | |
| Aluminum foil | Fisher Scientific | 01-213-103 | |
| Aluminum weighing dishes | Fisher Scientific | 08-732-102 | Diameter 66 mm |
| Bel-Art Vacuum Desiccator | Fisher Scientific | 08-594-15B | |
| Corning Costar TC-Treated Multiple Well Plates | Millipore Sigma | CLS3527-100EA | 24-well plate, polystyrene |
| Cyanoacrylate Adhesive | LocTite | N/A | |
| Digital Microscope | Keyence | VHX-S750E | |
| Disco DAD3350 Dicing Saw | Disco | DAD3350 | Used to cut silicon wafer into 1 cm x 1 cm samples |
| Double-Sided Polyimide Tape | Kapton Tape | PPTDE-1/4 | ¼” x 36 yds. |
| EP21LVMed – low viscosity, two component epoxy compound | Masterbond | EP21LVMed | Meets USP Class VI certification, Passes ISO 10993-5 for cytotoxicity |
| Epilog Fusion Pro 48 Laser Machine | Epilog | N/A | CO2 laser |
| Foam tape | XFasten | N/A | 1/8" Thick |
| Gamry Interface 1010E Potentiostat | Gamry | 992-00129 | |
| High precision 45° curved tapered very fine point tweezers/forceps | Fisher Scientific | 12-000-131 | |
| Lab tape | Fisher Scientific | 15-901-10L | |
| Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP) | EMD Millipore | 475870-25MG | Solid, stored at -20 °C |
| N-Hydroxysulfosuccinimide sodium salt, ≥98% (HPLC) | Sigma-Aldrich | 56485-250MG | Solid, stored desiccated at 4°C |
| Platinum clad niobium mesh anode | Technic | N/A | Clad with 125μ” of platinum on one side, framed in titanium with (1) 1” x 6” titanium strap centered on one 6” dimension |
| Silicon Planar Microelectrode Array, 16 Channel | NeuroNexus | A1x16-3mm-100-177-CM16LP | Electrode site material is iridium, shank thickness is 15 μm |
| Silicon Wafer | University Wafer | 1575 | Diameter 100 mm, p-type, boron-doped, 100 oriented, resistivity 0.01-0.02 Ohm-cm, thickness 525 um, single side polished, prime grade |
| Silver/silver Chloride reference electrode | Gamry Instruments | 930-00015 | |
| Solidworks | N/A | ||
| Stainless Steel Phillips Flat Head Screws | McMaster Carr | 96877A629 | #8-32, 1 1/2", fully threaded |
| Type I deionized water | ChemWorld | CW-DI1-20 | |
| Ultimaker 3 3D printer | Ultimaker | N/A | |
| Ultimaker Cura | Ultimaker | N/A | 3D printing software |
| Ultimaker NFC ABS Filament | Dynamism, Inc. | 1621 | 2.85 mm |
| Ultimaker NFC PLA Filament | Dynamism, Inc. | 1609 | 2.85 mm |
| Vacuum Gauge Vacuum Gauge | Measureman Direct | N/A | Glycerin Filled, 2-1/2” Dial Size, ¼”NPT, -30” Hg/-100kpa-0 |
| Wing nuts | Everbilt | 934917 | #8-32, zinc plated |
References
- Donoghue, J. Bridging the brain to the world: A perspective on neural interface systems. Neuron. 60 (3), 511-521 (2008).
- Ajiboye, A. B., et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. The Lancet. 398 (10081), 1821-1830 (2017).
- Ereifej, E. S., et al. Neural engineering: the process, applications, and its role in the future of medicine. Journal of Neural Engineering. 16 (6), 063002 (2019).
- Nicolas-Alonso, L. F., Gomez-Gil, J. Brain computer interfaces, a review. Sensors (Basel). 12 (2), 1211-1279 (2012).
- Leuthardt, E. C., Schalk, G., Moran, D., Ojemann, J. G. The emerging world of motor neuroprosthetics: a neurosurgical perspective. Neurosurgery. 59 (1), 1-14 (2006).
- Barrese, J. C., et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. Journal of Neural Engineering. 10 (6), 066014 (2013).
- Jorfi, M., Skousen, J. L., Weder, C., Capadona, J. R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. Journal of Neural Engineering. 12 (1), 011001 (2015).
- Prasad, A., et al. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. Journal of Neural Engineering. 9 (5), 056015 (2012).
- Hermann, J. K., Capadona, J. R. Understanding the role of innate immunity in the response to intracortical microelectrodes. Critical Reviews in Biomedical Engineering. 46 (4), 341-367 (2018).
- Ravikumar, M., et al. The roles of blood-derived macrophages and resident microglia in the neuroinflammatory response to implanted intracortical microelectrodes. Biomaterials. 35 (28), 8049-8064 (2014).
- Sawyer, A. J., et al. The effect of inflammatory cell-derived MCP-1 loss on neuronal survival during chronic neuroinflammation. Biomaterials. 35 (25), 6698-6706 (2014).
- Prasad, A., Sanchez, J. C. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. Journal of Neural Engineering. 9 (2), 026028 (2012).
- Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y., Purcell, E. K. Glial responses to implanted electrodes in the brain. Nature Biomedical Engineering. 1 (11), 862-877 (2017).
- McConnell, G. C., et al. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. Journal of Neural Engineering. 6 (5), 056003 (2009).
- Rennaker, R. L., Miller, J., Tang, H., Wilson, D. A. Minocycline increases quality and longevity of chronic neural recordings. Journal of Neural Engineering. 4 (2), 1-5 (2007).
- Carnicer-Lombarte, A., Chen, S. T., Malliaras, G. G., Barone, D. G. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. Frontiers in Bioengineering and Biotechnology. 9, 622524 (2021).
- Roitbak, T., Sykova, E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 28 (1), 40-48 (1999).
- Polikov, V. S., Tresco, P. A., Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. Journal of Neuroscience Methods. 148 (1), 1-18 (2005).
- Cui, X., Martin, D. C. Electrochemical deposition and characterization of poly(3,4-ethylenedioxythiophene) on neural microelectrode arrays. Sensors and Actuators B: Chemical. 89 (1), 92-102 (2003).
- Ludwig, K. A., Uram, J. D., Yang, J., Martin, D. C., Kipke, D. R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. Journal of Neural Engineering. 3 (1), 59-70 (2006).
- Keefer, E. W., Botterman, B. R., Romero, M. I., Rossi, A. F., Gross, G. W. Carbon nanotube coating improves neuronal recordings. Nature Nanotechnology. 3 (7), 434-439 (2008).
- Kim, D. -. H., Wiler, J. A., Anderson, D. J., Kipke, D. R., Martin, D. C. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomaterialia. 6 (1), 57-62 (2010).
- He, W., McConnell, G. C., Bellamkonda, R. V. Nanoscale laminin coating modulates cortical scarring response around implanted silicon microelectrode arrays. Journal of Neural Engineering. 3 (4), 316-326 (2006).
- Azemi, E., Lagenaur, C. F., Cui, X. T. The surface immobilization of the neural adhesion molecule L1 on neural probes and its effect on neuronal density and gliosis at the probe/tissue interface. Biomaterials. 32 (3), 681-692 (2011).
- Zhong, Y., Bellamkonda, R. V. Controlled release of anti-inflammatory agent alpha-MSH from neural implants. Journal of Controlled Release. 106 (3), 309-318 (2005).
- Shoffstall, A. J., et al. Potential for thermal damage to the blood-brain barrier during craniotomy: implications for intracortical recording microelectrodes. Journal of Neural Engineering. 15 (3), 034001 (2018).
- Bedell, H. W., et al. Understanding the effects of both CD14-meditated innate immunity and device/tissue mechanical mismatch in the neuroinflammatory response to intracortical microelectrodes. Frontiers in Neuroscience. 12, 772 (2018).
- Nguyen, J. K., et al. Influence of resveratrol release on the tissue response to mechanically adaptive cortical implants. Acta Biomaterialia. 29, 81-93 (2016).
- Sridharan, A., Nguyen, J. K., Capadona, J. R., Muthuswamy, J. Compliant intracortical implants reduce strains and strain rates in brain tissue in vivo. Journal of Neural Engineering. 12 (3), 036002 (2015).
- Nguyen, J. K., et al. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. Journal of Neural Engineering. 11, 056014 (2014).
- Harris, J. P., et al. In vivo deployment of mechanically adaptive nanocomposites for intracortical microelectrodes. Journal of Neural Engineering. 8 (4), 046010 (2011).
- Shoffstall, A. J., et al. Characterization of the neuroinflammatory response to Thiol-ene/Acrylate shape memory polymer coated intracortical microelectrodes. Micromachines. 10, 486 (2018).
- Simon, D. M., et al. Design and demonstration of an intracortical probe technology with tunable modulus. Journal of Biomedical Materials Research. Part A. 105 (1), 159-168 (2017).
- Ravikumar, M., et al. The effect of residual endotoxin contamination on the neuroinflammatory response to sterilized intracortical microelectrodes. Journal of Materials Chemistry. B. 2 (17), 2517-2529 (2014).
- Ecker, M., et al. Sterilization of thiol-ene/acrylate based shape memory polymers for biomedical applications. Macromolecular Materials and Engineering. 302 (2), 160331 (2017).
- Ereifej, E. S., et al. Implantation of neural probes in the brain elicits oxidative stress. Frontiers in Bioengineering and Biotechnology. 6 (9), 1-12 (2018).
- Potter, K. A., et al. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microelectrodes. Biomaterials. 34 (29), 7001-7015 (2013).
- Potter, K. A., et al. Curcumin-releasing mechanically adaptive intracortical implants improve the proximal neuronal density and blood-brain barrier stability. Acta Biomaterialia. 10 (5), 2209-2222 (2014).
- Potter-Baker, K. A., Capadona, J. R. Reducing the "stress": Antioxidative therapeutic and material approaches may prevent intracortical microelectrode failure. ACS Macro Letters. 4 (3), 275-279 (2015).
- Potter-Baker, K. A., et al. Development of superoxide dismutase mimetic surfaces to reduce accumulation of reactive oxygen species for neural interfacing applications. Journal of Materials Chemistry B. 2 (16), 2248-2258 (2014).
- Potter-Baker, K. A., et al. Implications of chronic daily antioxidant administration on the inflammatory response to intracortical microelectrodes. Journal of Neural Engineering. 12 (4), 046002 (2015).
- Kim, Y., et al. Ventricular delivery of resveratrol improves microelectrode recording performance and reduces oxidative stress. Micromachines. 12, 1446 (2021).
- Deku, F., et al. Amorphous silicon carbide ultramicroelectrode arrays for neural stimulation and recording. Journal of Neural Engineering. 15 (1), 016007 (2018).
- Ereifej, E. S., et al. The neuroinflammatory response to nanopatterning parallel grooves into the surface structure of intracortical microelectrodes. Advanced Functional Materials. 28 (12), 1704420 (2018).
- Kim, Y., et al. Nano-architectural approaches for improved intracortical interface technologies. Frontiers in Neuroscience. 12, 456 (2018).
- Mahajan, S., et al. Towards standardization of electrophysiology and computational tissue strain in rodent intracortical microelectrode models. Frontiers in Bioengineering and Biotechnology. 8, 416 (2020).
- Suresh, M. V., et al. The protective role of MnTBAP in oxidant-mediated injury and inflammation in a rat model of lung contusion. Surgery. 154 (5), 980-990 (2013).
- Liu, D., Shan, Y., Valluru, L., Bao, F. Mn (III) tetrakis (4-benzoic acid) porphyrin scavenges reactive species, reduces oxidative stress, and improves functional recovery after experimental spinal cord injury in rats: comparison with methylprednisolone. BMC Neuroscience. 14 (1), 23 (2013).
- Munief, W. M., et al. Silane deposition via gas-phase evaporation and high-resolution surface characterization of the ultrathin siloxane coatings. Langmuir. 34 (35), 10217-10229 (2018).
- Hoogerwerf, A. C., Wise, K. D. A three-dimensional microelectrode array for chronic neural recording. IEEE Transactions on Biomedical Engineering. 41 (12), 1136-1146 (1994).
- Staros, J. V., Wright, R. W., Swingle, D. M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Analalytical Biochemistry. 156 (1), 220-222 (1986).
- Yuan, X., Wolf, N., Mayer, D., Offenhausser, A., Wordenweber, R. Vapor-phase deposition and electronic characterization of 3-Aminopropyltriethoxysilane self-assembled monolayers on silicon dioxide. Langmuir. 35 (25), 8183-8190 (2019).
- Montgomery, D. C. . Design and Analysis of Experiments. Eighth edition. , (2013).
- Shoffstall, A. J., Capadona, J. R. Bio-inspired materials and systems for neural interfacing. Current Opinions in Biomedical Engineering. 6, 110-119 (2018).
- Skousen, J. L., Tresco, P. A. . Neuroprosthetics. Theory and Practice 2nd Edition. , 259-299 (2017).
- Michelson, N. J., et al. multi-modal analysis uncovers complex relationship at the brain tissue-implant neural interface: new emphasis on the biological interface. Journal of Neural Engineering. 15 (3), 033001 (2018).
- Hofmann, U. G., Capadona, J. R. Editorial: Bridging the gap in neuroelectronic interfaces. Frontiers in Neuroscience. 14, 457 (2020).
- Usoro, J., Sturgill, B., Musselman, K., Capadona, J. R., Pancrazio, J. J. On the definition of ‘chronic’ for intracortical microelectrode array applications. Micromachines. 12 (8), 972 (2021).
- Thompson, C. H., Saxena, A., Heelan, N., Salatino, J., Purcell, E. K. Spatiotemporal patterns of gene expression around implanted silicon electrode arrays. Journal of Neural Engineering. 18 (4), 1741 (2021).
- Golabchi, A., Woeppel, K. M., Li, X., Lagenaur, C. F., Cui, X. T. Neuroadhesive protein coating improves the chronic performance of neuroelectronics in mouse brain. Biosensors and Bioelectronics. 155, 112096 (2020).
- Zheng, X. S., et al. A superoxide scavenging coating for improving tissue response to neural implants. Acta Biomaterialia. 99, 72-83 (2019).
- Lee, H. C., et al. Foreign body response to intracortical microelectrodes is not altered with dip-coating of Polyethylene Glycol (PEG). Frontiers in Neuroscience. 11, 513 (2017).
- Boehler, C., et al. Actively controlled release of Dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials. 129, 176-187 (2017).
- Hess, A. E., et al. Development of a stimuli-responsive polymer nanocomposite toward biologically optimized, MEMS-based neural probes. Journal of Micromechanics and Microengineering. 21 (5), 054009 (2011).

