小鼠胚胎中早期心脏祖细胞的实时成像
Summary
我们提出了小鼠胚胎培养和成像的详细方案,可实现心脏祖细胞的3D +时间成像。该视频工具包解决了成功进行实时成像所需的关键技能,否则很难从纯文本出版物中获得。
Abstract
心脏发育的第一步意味着细胞行为和分化的剧烈变化。虽然对固定胚胎的分析允许在静止快照中详细研究特定的发育阶段,但实时成像通过在胚胎发育过程中成像来捕获动态形态发生事件,例如细胞迁移、形状变化和分化。这补充了固定分析,并扩展了对胚胎发生过程中器官如何发育的理解。尽管具有优势,但由于其技术挑战,实时成像很少用于小鼠模型。早期小鼠胚胎在 离体 培养时很敏感,需要有效处理。为了促进实时成像在小鼠发育研究中的更广泛应用,本文提出了一种双光子实时显微镜的详细方案,该协议允许在小鼠胚胎中长期采集。除方案外,还提供了有关胚胎处理和培养优化的提示。这将有助于了解早期小鼠器官发生的关键事件,增强对心血管祖细胞生物学的理解。
Introduction
心脏在胚胎发生早期形成,开始向整个胚胎输送营养物质,同时继续发育1。在小鼠胚胎中,原肠胚形成开始一天半后,一个基本的心脏器官在前极2,3处组装。到早期条纹(ES)阶段,外胚层中的心脏祖细胞通过原始条纹进入新生的中胚层4,5,6并开始迁移到前极,在那里它们分化形成原始心脏管。在整个过程中,除了迁移7之外,早期心脏祖细胞还经历细胞重排,形状转换和分化(图1)。
近一个世纪以来,早期的心脏祖细胞因其同时分化和构建功能器官的非凡能力而吸引了研究人员。在过去的二十年中,克隆分析和条件敲除模型表明,早期心脏发育涉及高度动态过程中的不同细胞来源8,9,10。然而,原始心脏管的3D结构及其形态发生的动态性质使其研究具有挑战性(图1),我们远未了解其全部复杂性11。
为了研究这些动态细胞过程,实时成像方法现在提供了前所未有的细节7,12,13,14。在小鼠模型中,实时方法是询问静态分析难以解决的发展主题的关键7,13,15。虽然长期离体培养和强大的显微镜装置正在快速发展16,17,但很少有研究人员具备成功对活胚胎进行成像的专业知识。尽管纸质出版物提供了足够的技术细节来重现实时成像实验,但如果没有视觉示例或点对点帮助,一些技能和技巧很难掌握。为了加速这一学习过程并在实验室中推广实时成像的使用,我们组装了一个视频协议(图2),该协议收集了对原肠胚小鼠胚胎进行实时成像的必要技能。
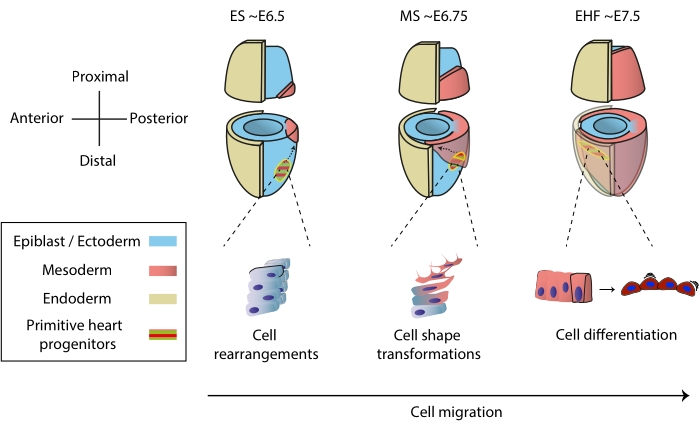
图1:小鼠胚胎中心脏祖细胞的早期分化,从原肠胚形成开始到原始心脏管形成之前的阶段。 心脏祖细胞在原肠胚形成开始后不久进入中胚层,迁移到胚胎的另一侧。形态和胚胎日(E)阶段写在图表的顶部。虚线箭头描绘了原肠胚形成过程中原始心脏管祖细胞的迁移轨迹。这个数字改编自11。缩写:ES = 早期连胜;MS = 中间条纹;EHF = 早期头部折叠。 请点击此处查看此图的大图。
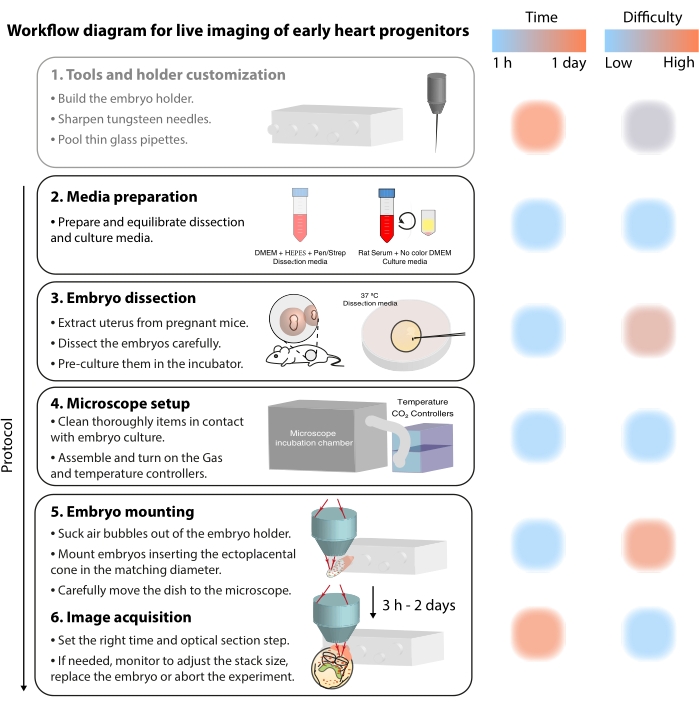
图 2:早期心脏祖细胞实时成像的工作流程图。 请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
早期的心脏祖细胞组织在一个原始的心脏管中,该管在仍在形成时就开始跳动。了解这一过程是如何发生的,是将广泛的先天性心脏缺陷确定为特定形态发生事件的关键。为此,实时成像提供了一个机会来研究正常和有缺陷的胚胎发育,并提高时间分辨率。这对于研究早期心脏祖细胞特别有用,因为它们通过多种分化和迁移行为快速过渡7。
由于我们可以在?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
作者感谢Kenzo Ivanovitch博士以前在这种方法上的工作,以及Shigenori Nonaka博士(日本国立自然科学研究所)的小组提供了胚胎安装的初步专业知识。这项研究得到了西班牙科学和创新部的Grant PGC2018-096486-B-I00和欧盟地平线2020计划的Grant H2020-MSCA-ITN-2016-722427和FEDER Andalucía 2014-2020运营计划的MT和赠款1380918的支持。MS得到了La Caixa基金会博士奖学金(LCF / BQ / DE18 / 11670014)和生物学家公司旅行奖学金(DEVTF181145)的支持。CNIC得到了西班牙科学部和ProCNIC基金会的支持。
Materials
| #55 Forceps | Dumont | 11295-51 | |
| 35 mm Dish with glass coverslip bottom 14 mm Diameter | Mattek | P35G-1.5-14-C | |
| 35 mm vise table | Grandado | SKU 8798771617573 | |
| 50 mL tubes | BD Falcon | 352070 | |
| Distilled water | |||
| DMEM – Dulbecco's Modified Eagle Medium | Gibco | 11966025 | with L-Glutamine, without Glucose, without Na Pyruvate |
| Fetal Bovine Serum | Invitrogen | 10438-026 | |
| Fluorescent reporter transgenic mice (Tg(CBF:H2BVenus,+) | JAX | ||
| Fluorobrite DMEM | ThermoFisher | A1896701 | DMEM for live-cell imaging |
| High-vacuum silicone grease | Dow Corning | Z273554-1EA | |
| Holder for wires | Perlen Pressen | pwb1 | |
| LSM 780 Upright microscope | Zeiss | ||
| MaiTai Deepsee far red pulsed-laser tuned at 980 nm | Spectra-Physics | ||
| Non Descanned Detectors equipped with the filter sets cyan-yellow (BP450-500/BP520-560), green-red (BP500-520/BP570-610) and yellow-red (BP520-560/BP645-710) |
Zeiss | ||
| Obj: 20x water dipping 1.0 NA, long working distance | Zeiss | ||
| P1000 and P200 pipettes | |||
| Paraffin Oil | Nidacon | VNI0049 | |
| Penicillin-streptomycin | Invitrogen | 15070-063 | (the final concentration should be 50 μg/mL penicillin and 50 μg/mL streptomycin) |
| Petri dishes 35 mm x 10 mm | BD Falcon | 351008 | |
| Pipette tips | |||
| Polymethyl methacrylate | Reused from old laboratory equipment | ||
| Rat Serum culture embryo, male rats SPRAGUE DAWLEY RjHan SD | Janvier Labs | 9979 | |
| Set of 160 mm fines | RS PRO | 541-6933 | |
| Standard 1.0 mm glass capillaries | Anima Lab | 1B100F-3 | |
| Sterile 0.22 μm syringe filter | Corning | 431218 | |
| Sterile 5 mL syringe | Fisher Scientific | 15809152 | |
| Tungsten needles | |||
| Ultrasonic homogeniser (sonicator) | Bandelin | BASO_17021 |
References
- Tyser, R. C. V., et al. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. eLife. 5, 17113 (2016).
- Kelly, R. G., Buckingham, M. E., Moorman, A. F. Heart fields and cardiac morphogenesis. Cold Spring Harbor Perspectives in Medicine. 4 (10), 015750 (2014).
- Evans, S. M., Yelon, D., Conlon, F. L., Kirby, M. L. Myocardial lineage development. Circulation Research. 107 (12), 1428-1444 (2010).
- Tam, P. P., Parameswaran, M., Kinder, S. J., Weinberger, R. P. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 124 (9), 1631-1642 (1997).
- Kinder, S. J., Loebel, D. A. F., Tam, P. P. L. Allocation and early differentiation of cardiovascular progenitors in the mouse embryo. Trends in Cardiovascular Medicine. 11 (5), 177-184 (2001).
- Lawson, K. A. Fate mapping the mouse embryo. International Journal of Developmental Biology. 43 (7), 773-775 (1999).
- Ivanovitch, K., Temiño, S., Torres, M. Live imaging of heart tube development in mouse reveals alternating phases of cardiac differentiation and morphogenesis. eLife. 6, 30668 (2017).
- Meilhac, S. M., Buckingham, M. E. The deployment of cell lineages that form the mammalian heart. Nature Reviews Cardiology. 15 (11), 705-724 (2018).
- Buckingham, M., Meilhac, S., Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nature Reviews Genetics. 6 (11), 826-835 (2005).
- Meilhac, S. M., Lescroart, F., Blanpain, C. D., Buckingham, M. E. Cardiac cell lineages that form the heart. Cold Spring Harbor Perspectives in Medicine. 4 (9), 013888 (2014).
- Sendra, M., Domínguez, J. N., Torres, M., Ocaña, O. H. Dissecting the complexity of early heart progenitor cells. Journal of Cardiovascular Development and Disease. 9 (1), 5 (2022).
- McDole, K., et al. In toto imaging and reconstruction of post-implantation mouse development at the single-cell level. Cell. 175 (3), 859-876 (2018).
- Saykali, B., et al. Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. eLife. 8, 42434 (2019).
- Ichikawa, T., et al. Live imaging of whole mouse embryos during gastrulation: Migration analyses of epiblast and mesodermal cells. PLoS ONE. 8 (7), 64506 (2013).
- Tyser, R. C. V., et al. Single-cell transcriptomic characterization of a gastrulating human embryo. Nature. 600 (7888), 285-289 (2021).
- Aguilera-Castrejon, A., et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature. 593 (7857), 119-124 (2021).
- Yue, Y., et al. in toto live imaging of cardiomyocyte behaviour during mouse ventricle chamber formation at single-cell resolution. Nature Cell Biology. 22 (3), 332-340 (2020).
- Nowotschin, S., Xenopoulos, P., Schrode, N., Hadjantonakis, A. K. A bright single-cell resolution live imaging reporter of Notch signaling in the mouse. BMC Developmental Biology. 13 (1), 15 (2013).
- Cold Spring Harbor Protocols. Sharpened tungsten needles. Cold Spring Harbor Protocols. , (2012).
- Tam, P. P., Snow, M. H. The in vitro culture of primitive-streak-stage mouse embryos. Journal of Embryology and Experimental Morphology. 59, 131-143 (1980).
- Garcia, M. D., Udan, R. S., Hadjantonakis, A. K., Dickinson, M. E. Preparation of rat serum for culturing mouse embryos. Cold Spring Harbor Protocols. 2011 (4), 5593 (2011).
- Tam, P. P. L. Postimplantation mouse development: Whole embryo culture and micro- manipulation. International Journal of Developmental Biology. 42 (7), 895-902 (1998).
- Optimización de propiedades fisicoquímicas y medios de cultivo para el cultivo del embrión de ratón ex vivo. Universidad de Jaén. Biología Experimental Available from: https://hdl.handle.net/10953.1/1400 (2021)
- Behringer, R., Gertsenstein, M., Vintersen Nagy, K., Nagy, A. . Manipulating the mouse embryo: A laboratory manual, Fourth Edition. , 814 (2014).
- Shea, K., Geijsen, N. Dissection of 6.5 dpc mouse embryos. Journal of Visualized Experiments. (2), e160 (2006).
- Nonaka, S. Modification of mouse nodal flow by applying artificial flow. Methods in Cell Biology. 91, 287-297 (2009).
- Garcia, M. D., Udan, R. S., Hadjantonakis, A. K., Dickinson, M. E. Time-lapse imaging of postimplantation mouse embryos. Cold Spring Harbor Protocols. 2011 (4), 5595 (2011).
- Crainiciuc, G., et al. Behavioural immune landscapes of inflammation. Nature. 601 (7893), 415-421 (2022).

