Creación de la columna de Winogradsky: Un método que sirve para enriquecer las especies microbianas en una muestra de sedimento
English
Share
Overview
Fuente: Elizabeth Suter1, Christopher Corbo1, Jonathan Blaize1
1 Departamento de Ciencias Biológicas, Wagner College, 1 Campus Road, Staten Island NY, 10301
La columna Winogradsky es un ecosistema en miniatura y cerrado utilizado para enriquecer las comunidades microbianas de sedimentos, especialmente las que participan en el ciclismo de azufre. La columna fue utilizada por primera vez por Sergei Winogradsky en la década de 1880 y desde entonces se ha aplicado en el estudio de muchos microorganismos diversos involucrados en la biogeoquímica, tales como fotocontautorátesis, oxidantes de azufre, reductores de sulfato, metanogenos, oxidantes de hierro, nitrógeno ciclistas, y más (1,2).
La mayoría de los microorganismos en la Tierra se consideran inculturables,lo que significa que no pueden aislarse en un tubo de ensayo o en una placa de petri (3). Esto es debido a muchos factores, incluyendo que los microorganismos dependen de otros para ciertos productos metabólicos. Las condiciones en una columna de Winogradsky imitan de cerca el hábitat natural de un microorganismo, incluyendo sus interacciones con otros organismos, y permite que se cultivan en un laboratorio. Por lo cual, esta técnica permite a los científicos estudiar estos organismos y entender cómo son importantes para los ciclos biogeoquímicos de la Tierra sin tener que cultivarlos de forma aislada.
Los ambientes de la Tierra están llenos de microorganismos que prosperan en todo tipo de hábitats, como suelos,agua oceánica, nubes y sedimentos de aguas profundas. En todos los hábitats, los microorganismos dependen unos de otros. A medida que un microorganismo crece, consume sustratos particulares,incluyendo combustibles ricos en carbono como azúcares, así como nutrientes, vitaminas y gases respiratorios como el oxígeno. Cuando estos recursos importantes se agotan, diferentes microorganismos con diferentes necesidades metabólicas pueden entonces florecer y prosperar. Por ejemplo, en la columna Winogradsky, los microbios consumen primero el material orgánico añadido mientras agotan el oxígeno en las capas inferiores de la columna. Una vez que se agota el oxígeno, los organismos anaeróbicos pueden tomar el control y consumir diferentes materiales orgánicos. Este desarrollo consecutivo de diferentes comunidades microbianas a lo largo del tiempo se denomina sucesión (4). La sucesión microbiana es importante en una columna de Winogradsky, donde la actividad microbiana cambia la química del sedimento, que luego afecta la actividad de otros microbios y así sucesivamente. Muchos microorganismos en suelos y sedimentos también viven a lo largo de gradientes,que son zonas de transición entre dos tipos diferentes de hábitats basados en las concentraciones de sustratos (5). En el punto correcto en el gradiente, un microbio puede recibir cantidades óptimas de diferentes sustratos. A medida que se desarrolla una columna Winogradsky, comienza a imitar estos gradientes naturales, particularmente en oxígeno y sulfuro (Fig. 1).
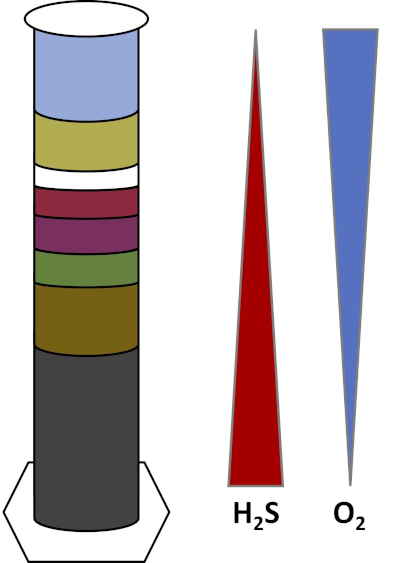
Figura 1: Representación de los gradientes de oxígeno (O2)y sulfuro (H2S) que se desarrollan en una columna Winogradsky.
En una columna de Winogradsky, el barro y el agua de un estanque o humedal se mezclan en una columna transparente y se les permite incubar, normalmente en la luz. Se añaden sustratos adicionales a la columna para dar a la comunidad fuentes de carbono, generalmente en forma de celulosa, y azufre. Los fotosintetizadores suelen comenzar a crecer en las capas superiores del sedimento. Estos microorganismos fotosintéticos se componen en gran medida de cianobacterias,que producen oxígeno y aparecen como una capa verde o marrón-marrón (Fig. 2, Tabla 1). Mientras que la fotosíntesis produce oxígeno, el oxígeno no es muy soluble en agua y disminuye por debajo de esta capa (Fig. 1). Esto crea un gradiente de oxígeno, que va desde altas concentraciones de oxígeno en las capas superiores hasta cero oxígeno en las capas inferiores. La capa oxigenada se llama la capa aeróbica y la capa sin oxígeno se llama la capa anaeróbica.
En la capa anaeróbica, muchas comunidades microbianas diferentes pueden proliferar dependiendo del tipo y la cantidad de sustratos disponibles, la fuente de los microbios iniciales y la porosidad del sedimento. En la parte inferior de la columna, los organismos que descomponen anaeróbicamente la materia orgánica pueden prosperar. La fermentación microbiana produce ácidos orgánicos a partir de la descomposición de la celulosa. Estos ácidos orgánicos pueden ser utilizados por reductoresde sulfato, que oxidan esos orgánicos usando sulfato, y producen sulfuro como subproducto. La actividad de los reductores de sulfato se indica si el sedimento se vuelve negro, porque el hierro y el sulfuro reaccionan para formar minerales de sulfuro de hierro negro (Fig. 2, Tabla 1). El sulfuro también se difunde hacia arriba, creando otro gradiente en el que las concentraciones de sulfuro son altas en la parte inferior de la columna y bajas en la parte superior de la columna (Fig. 1).
Cerca del centro de la columna, los oxidantes de azufre aprovechan el suministro de oxígeno desde arriba y sulfuro desde abajo. Con la cantidad correcta de luz, los oxidantes fotosintéticos de azufre pueden desarrollarse en estas capas. Estos organismos se conocen como bacterias de azufre verde y púrpura,y a menudo aparecen como filamentos y manchas verdes, púrpuras o púrpura-rojos (Fig. 2, Tabla 1). Las bacterias de azufre verde tienen una mayor tolerancia al sulfuro y generalmente se desarrollan en la capa directamente debajo de las bacterias de azufre púrpura. Por encima de las bacterias de azufre púrpura, también se pueden desarrollar bacterias de nonsulfurpúrpura. Estos organismos fotosintetizan utilizando ácidos orgánicos como donantes de electrones en lugar de sulfuro y a menudo aparecen como una capa roja, púrpura, naranja o marrón. Los oxidantes de azufre no fotosintéticos pueden desarrollarse por encima de las bacterias púrpuras nonsulfur, y estos generalmente aparecen como filamentos blancos (Fig. 2, Tabla 1). Además, también se pueden formar burbujas en la columna Winogradsky. Las burbujas en las capas aeróbicas indican la producción de oxígeno por las cianobacterias. Las burbujas en las capas anaeróbicas son probablemente debido a la actividad de los metanogenos, organismosque descomponen anaeróbicamente la materia orgánica y forman metano como subproducto.
| Posición en la columna | Grupo funcional | Ejemplos de organismos | Indicador visual |
| Arriba | Fotosíntesis | Cianobacterias | Capa verde o marrón rojizo. A veces burbujas de oxígeno. |
| Oxidantes de azufre no fotosintéticos | Beggiatoa, Thiobacilus | Capa blanca. | |
| Bacterias púrpuras sin azufre | Rhodomicrobium, Rhodospirilum, Rhodopseuodmonas | Capa roja, púrpura, naranja o marrón. | |
| Bacterias de azufre púrpura | Chromatium | Capa púrpura, o púrpura-roja. | |
| Bacterias de azufre verde | Clorobio | Capa verde. | |
| Bacterias reductoras de sulfato | Desulfovibrio, Desulfotomaculum, Desulfobacter, Desulfuromonas | Capa negra. | |
| Parte inferior | Metanógenos | Methanococcus, Methanosarcina | A veces burbujas de metano. |
Tabla 1: Los principales grupos de bacterias que pueden aparecer en una columna clásica de Winogradsky, de arriba abajo. Se dan ejemplos de organismos de cada grupo, y se enumeran los indicadores visuales de cada capa de organismos. (2002) y Rogan et al. (2005).
Procedure
Results
In this experiment, water and sediment were collected from a freshwater habitat. Two Winogradsky columns were constructed and allowed to develop: a classical Winogradsky column incubated in the light at room temperature (Fig. 2A) and a Winogradsky column incubated in the dark at room temperature (Fig. 2B).

Figure 2B: A photo of classical Winogradsky column (left), incubated at room temperature in light for 68 days and a Winogradsky column incubated at room temperature in the dark for 68 days (right).
After allowing the columns to develop for 7-9 weeks, the layers in the classical column can be compared to the column incubated in the dark (Fig. 2B). In the classical Winogradsky column, a green cyanobacterial layer can be observed near the top of the tube. Near the center of the tube, a red-purple layer can be observed, indicative of purple nonsulfur bacteria. Under this layer, a purple-red layer is observed, indicative of purple sulfur bacteria. Directly under this layer, black sediment can be observed in the anaerobic region of the column, indicative of sulfate reducing bacteria.
The column grown in the dark (Fig. 2B) developed differently than the classical Winogradsky column. Like the classical column, the dark column yielded black sediment near the bottom of the column, indicative of sulfate reducing bacteria. The dark column did not yield the green cyanobacterial layer, nor the red, purple, or green layers indicative of purple nonsulfur, purple sulfur, and green sulfur bacteria, respectively. These groups are dependent on light for growth, and therefore unable to grow in the dark.
The precise results of each Winogradsky column will vary widely with their incubation conditions and their source habitats.
Microbial communities originating from freshwater habitats will not be accustomed to high salt concentrations and the addition of salt may slow down or inhibit growth. Conversely, there may be sufficient halophilic bacteria in brackish and saltwater habitats so that the addition of salts makes no difference or even enhances the growth of particular layers when compared to a column without added salts.
Sandy sediments are more porous than muddy sediments. If enough sulfide is produced in such porous sediments, sulfides can diffuse all the way to the top of the column and inhibit growth of aerobic organisms. In this case, the column may only contain layers indicative of anaerobes and may not contain any aerobes, such as the cyanobacteria.
Freshwater generally contains less sulfate than saltwater. Sulfate is important for the growth of sulfate-reducing bacteria. Sulfate reducers create sulfide as a byproduct and are indicated by the development of a black layer in the bottom of the column. If sulfate is not supplemented to freshwater communities, sulfate reducers may not produce enough sulfide. The creation of the sulfide byproduct is important for the growth of green and purple sulfur bacteria and the nonphotosynthetic sulfur oxidizers. In these cases, sulfur oxidizers can still grow using the egg yolk as a source of sulfur, even if the sulfate reducers (black layer) never develop.
Different wavelengths of light should select for organisms with different absorption pigments. A column kept in the dark will only allow for nonphotosynthetic organisms to grow, including sulfate reducers, iron oxidizers, and methanogens. Photosynthesizers have pigments that absorb light at different wavelengths within the visible range (~400-700nm). By covering a column with, for example, blue cellophane, blue light (~450-490nm) is blocked from entering the column. All of the photosynthesizers in the column have pigments which require the blue wavelengths (6) and their growth should be inhibited. On the other hand, red cellophane will block light of ~635-700nm. These wavelengths are important for the pigments used by cyanobacteria (6), while purple sulfur, green sulfur, and purple nonsulfur bacteria may still be able to grow.
Different microbial communities may have vastly different adaptive abilities to cope with changes in temperatures. High temperatures can enhance rates of microbial activity when sufficient thermophiles are present. On the other hand, in the absence of thermophiles, high temperatures may decrease overall microbial activity. Similarly, low temperatures may decrease overall microbial activity unless the microbial community contains sufficient psychrophiles.
Applications and Summary
The Winogradsky column is an example of an interdependent microbial ecosystem. After mixing mud, water, and additional carbon and sulfur substrates in a vertical column, the stratified ecosystem should stabilize into separate, stable zones over several weeks. These zones are occupied by different microorganisms which flourish at a particular spot along the gradient between the sulfide-rich sediment in the bottom and the oxygen-rich sediment at the top. By manipulating the conditions and substrates within the Winogradsky column, the presence and activity of different microorganisms such as halophiles, thermophiles, psychrophiles, sulfur oxidizers, sulfur reducers, iron oxidizers, and photosynthesizers can be observed.
References
- Zavarzin G. (2006). Winogradsky and modern microbiology. Microbiology 75(6): 501-511. doi: 10.1134/s0026261706050018
- Esteban DJ, Hysa B, and Bartow-McKenney C (2015). Temporal and Spatial Distribution of the Microbial Community of Winogradsky Columns. PLoS ONE 10(8): e0134588. doi:10.1371/journal.pone.0134588
- Lloyd KG, Steen AD, Ladau J, Yin J, and Crosby L. (2018). Phylogenetically novel uncultured microbial cells dominate Earth microbiomes. mSystems 3(5): e00055-18. doi:10.1128/mSystems.00055-18
- Anderson DC, and Hairston RV (1999). The Winogradsky Column & Biofilms: Models for Teaching Nutrient Cycling & Succession in an Ecosystem. The American Biology Teacher, 61(6): 453-459. doi: 10.2307/4450728
- Dang H, Klotz MG, Lovell CR and Sievert SM (2019) Editorial: The Responses of Marine Microorganisms, Communities and Ecofunctions to Environmental Gradients. Frontiers in Microbiology 10(115). doi: 10.3389/fmicb.2019.00115
- Stomp M, Huisman J, Stal LJ, and Matthijs HCP. (2007) Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME Journal. 1(4): 271-282. doi:10.1038/ismej.2007.59
- Perry JJ, Staley JT, and Lory S. (2002) Microbial Life, First Edition, published by Sinauer Associates
- Rogan B, Lemke M, Levandowsky M, and Gorrel T. (2005) Exploring the Sulfur Nutrient Cycle Using the Winogradsky Column. The American Biology Teacher, 67(6): 348-356. doi: 10.2307/4451860
Transcript
Most of the Earth’s microorganisms cannot be cultured in a lab, often because they rely on other microbes within their native communities. A Winogradsky column, named for its inventor Sergei Winogradsky, is a miniature, enclosed ecosystem which enriches the microbial communities within a sediment sample, enabling scientists to study many of the microbes that play a vital role in Earth’s biogeochemical processes, without needing to isolate and culture them individually.
Typically, mud and water from an ecosystem, such as a pond or a marsh, are mixed. As an optional experiment, salt can be added to this mixture to enrich various halophile species. Next, a small portion of the mixture is supplemented with carbon, usually in the form of cellulose from newspaper, and sulfur, usually from an egg yolk. For another optional experiment, a nail can be added to this mixture to enrich certain Gallionella species. This new mixture is then added to a transparent column, so that the column is one quarter full. Finally, the rest of the mud mixture and more water is added to the column until it is most of the way full.
Succession, which refers to the consecutive development of different microbial communities over time, can be observed in real time with a Winogradsky column. As microbes grow within the column, they consume specific substrates and change the chemistry of their environment. When their substrates are depleted, the original microbes die off and microbes with different metabolic needs can flourish in the altered environment. Over time, visibly distinct layers begin to form, each containing parts of a bacterial community with different microenvironmental needs.
For example, photosynthetic microbes, largely composed of cyanobacteria, form green or red-brown layers near the top of the column. Since photosynthesis produces oxygen, often seen as bubbles in the top portion of the column, a gradient is formed with the highest oxygen concentrations near the top, and the lowest towards the bottom. Depending upon the available substrates, different microbial communities can grow in the anaerobic bottom layer. Bubbles in this layer can indicate the presence of methanogens, which create methane gas via fermentation. Here, the microbial fermentation of cellulose results in organic acids. Sulfate reducers oxidize those acids to produce sulfide, and their activity is indicated by black sediment. Sulfide diffuses upward in the column, creating yet another gradient where sulfide concentrations are highest towards the bottom of the column, and lowest near the top. Towards the middle of the column, sulfur oxidizers utilize the oxygen from above and sulfide from below. With adequate light, photosynthetic sulfur oxidizers, such as green and purple sulfur bacteria, develop. Green sulfur bacteria tolerate higher sulfide concentrations. Thus, they grow directly below the purple sulfur bacteria. Directly above this layer, purple non-sulfur bacteria form a red-orange layer. Nonphotosynthetic sulfur oxidizers are indicated by the presence of white filaments.
Conditions such as light and temperature can also be varied to enrich other communities. In this video, you will learn how to construct a Winogradsky column, and vary the growing conditions and substrates to enrich specific microbial communities.
First, locate an appropriate aquatic ecosystem, such as a pond or marsh. The sediment samples should come from the area near the water’s edge, and be completely saturated with water. Then, use a shovel and a bucket to collect one to two liters of the saturated mud. Next, obtain approximately three liters of fresh water from the same source and return to the lab with the field samples.
In the lab, put on the appropriate personal protective equipment, including a lab coat and gloves. Now, transfer approximately 750 milliliters of mud to a mixing bowl. Then, sift through the mud to remove large rocks, twigs, or leaves and use a spoon to break apart any clumps. Next, add some of the fresh water to the mixing bowl, and stir with a large spoon. Add water until the consistency of the water-mud mixture is similar to a milkshake. Continue to make sure there are no clumps.
As an optional experiment, select for halophilic bacteria by adding 25 to 50 milligrams of salt to the mud mixture.
Then, transfer approximately 1/3 of the water-mud mixture to a second mixing bowl. Add one egg yolk and a handful of shredded newspaper to the bowl. Next, add this mixture to the column, until it is about 1/4 full. Next, add the water-mud mixture without the egg and newspaper to the column, until it is approximately 3/4 full. Then, add more water to the column, leaving a 1/2 inch space on top. Cover the column with plastic wrap and secure it with a rubber band.
Incubate the column in the light near a window at room temperature for the next four to eight weeks. Throughout the incubation period, monitor changes in the Winogradsky column at least once a week for the development of different colored layers and the formation of bubbles. Additionally, record the time it takes for different layers to develop.
Another modification that can be done is incubating the column near a radiator to select for thermophilic bacteria, or in a refrigerator to select for psychrophilic bacteria. Vary the light conditions by placing different columns in high light, low light, or darkness to incubate. Alternatively, limit the wavelength of incoming light by covering the column with different shades of cellophane to determine which colors select for different bacterial groups. For another optional experiment, to enrich iron-oxidizing bacteria, add a nail to the mud-water mixture prior to the addition of newspaper and an egg yolk.
After one to two weeks, growth of the cyanobacterial layer is indicated by a green or red-brown film on top of the mud layer of the classical Winogradsky column. Over time, the appearance and evolution of the different layers is monitored, each indicative of the different types of bacteria present. When comparing a column grown in the dark to a traditional Winogradsky column, we see the dark treatment yields the black layer at the bottom of the column, indicative of sulfate-reducing bacteria.
The dark column may also yield other layers, depending on other incubation conditions. Additionally, the dark column doesn’t yield the green cyanobacterial layer, nor the red, purple, or green layers indicative of purple non-sulfur, purple sulfur, and green sulfur bacteria respectively. These groups are dependent on light for growth.
