Protocol for the Synthesis of Ortho-trifluoromethoxylated Aniline Derivatives
Summary
An operationally simple procedure for the synthesis of ortho-trifluoromethoxylated aniline derivatives via a two-step sequence of O-trifluoromethylation of N-aryl-N-hydroxyacetamide followed by thermally induced intramolecular OCF3-migration is reported.
Abstract
Molecules bearing trifluoromethoxy (OCF3) group often show desired pharmacological and biological properties. However, facile synthesis of trifluoromethoxylated aromatic compounds remains a formidable challenge in organic synthesis. Conventional approaches often suffer from poor substrate scope, or require use of highly toxic, difficult-to-handle, and/or thermally labile reagents. Herein, we report a user-friendly protocol for the synthesis of methyl 4-acetamido-3-(trifluoromethoxy)benzoate using 1-trifluoromethyl-1,2-benziodoxol-3(1H)-one (Togni reagent II). Treating methyl 4-(N-hydroxyacetamido)benzoate (1a) with Togni reagent II in the presence of a catalytic amount of cesium carbonate (Cs2CO3) in chloroform at RT afforded methyl 4-(N-(trifluoromethoxy)acetamido)benzoate (2a). This intermediate was then converted to the final product methyl 4-acetamido-3-(trifluoromethoxy)benzoate (3a) in nitromethane at 120 °C. This procedure is general and can be applied to the synthesis of a broad spectrum of ortho-trifluoromethoxylated aniline derivatives, which could serve as useful synthetic building blocks for the discovery and development of new pharmaceuticals, agrochemicals, and functional materials.
Introduction
The trifluoromethoxy (OCF3) group has made a profound impact on life and materials science research since the first synthesis of trifluoromethyl ether in 1935.2 Due to its unique combination of high electronegativity (χ = 3.7)3 and excellent lipophilicity (Πx = 1.04),4 the trifluoromethoxy group has found broad applications in medicine, agriculture, and materials industry.5-10 However, facile introduction of the OCF3 group into organic molecules, especially aromatic compounds, remains a major challenge in synthetic chemistry.
Over the last few decades, efforts to address this challenge led to the development of a handful of transformations for the synthesis of trifluoromethoxylated arenes.5-7,9-11 These include (i) chlorine/fluorine exchange on trichlorinated precursors;1,12-17 (ii) deoxyfluorination of fluoroformates;18 (iii) oxidative fluorodesulfurization;19-21 (iv) electrophilic trifluoromethylation of alcohols;22-25 (v) nucleophilic trifluoromethoxylation;26-30, (vi) transition metal-mediated trifluoromethoxylation of aryl borates and stannanes;31 and (vii) radical trifluoromethoxylation.32,33 Nevertheless, many of these approaches either suffer from poor substrate scope or require use of highly toxic and/or thermally labile reagents. Therefore, due to the lack of a general and user-friendly method to synthesize OCF3-containing compounds, the potential of the OCF3 group has not been fully exploited in chemistry.
As part of our interest in trifluoromethoxylation reactions,34 we describe herein a two-step protocol (i.e., radical O-trifluoromethylation and thermally induced OCF3-migration) for the synthesis of methyl 4-acetamido-3-(trifluoromethoxy)benzoate (3a) from methyl 4-(N-hydroxyacetamido)benzoate (1a). The strategy is easy-to-operate and applicable to the synthesis of a wide range of ortho-trifluoromethoxylated aniline derivatives.
Protocol
1. Precursor Preparation: Synthesis of Methyl 4-(N-hydroxyacetamido)benzoate (1a)
- Reduction of methyl 4-nitrobenzoate.
- Add 5.00 g of methyl 4-nitrobenzoate (27.6 mmol, 1.00 equiv), 159 mg of 5% Rhodium on carbon (Rh/C, 0.300 mol% Rh), and a magnetic stir-bar into an oven-dried 250 ml two-neck round-bottom flask (dried at 150 °C for 18 hr).
NOTE: Reagents can be weighed out under ambient atmosphere. However, the reaction needs to be carried out under nitrogen atmosphere. - Connect one neck of the flask to a nitrogen/vacuum manifold and cap the other neck with a septum. Perform three vacuum-refill cycles (i.e., pumping the air out of the flask and replacing the resulting vacuum with nitrogen gas) to replace the air in the flask with nitrogen gas.
- Add 138 ml anhydrous tetrahydrofuran (THF, 0.200 M) to the reaction flask using airtight syringe. Cool and stir the reaction mixture at 0 °C for 15 min.
- Add 1.47 ml of hydrazine monohydrate (1.52 g, 30.4 mmol, 1.20 equiv) dropwise to the reaction mixture at 0 °C using an airtight syringe. Monitor the reaction using a thin layer chromatography (TLC). Use hexanes:ethyl acetate (EtOAc) (4:1 v/v, Rf = 0.23) as an eluent to develop the TLC.
- When methyl 4-nitrobenzoate is completely consumed, filter the reaction mixture through a short pad of diatomaceous earth (i.e., Celite, 5 g) in a 60 ml frit Buchner funnel using vacuum filtration. Wash the filter with EtOAc (20 ml x 3 times). Concentrate the filtrate in vacuo using a rotary evaporator to afford the crude methyl 4-(N-hydroxyamino)benzoate, which is used directly without further purification.
- Add 5.00 g of methyl 4-nitrobenzoate (27.6 mmol, 1.00 equiv), 159 mg of 5% Rhodium on carbon (Rh/C, 0.300 mol% Rh), and a magnetic stir-bar into an oven-dried 250 ml two-neck round-bottom flask (dried at 150 °C for 18 hr).
- Acetyl protection of methyl 4-(N-hydroxyamino)benzoate
- Add 2.55 g of sodium bicarbonate (NaHCO3, 30.4 mmol, 1.20 equiv), all the crude methyl 4-(N-hydroxyamino)benzoate obtained from the previous step, and a stir-bar into an oven-dried 500 ml two-neck round-bottom flask.
- Cap one neck with a septum and connect another neck to a nitrogen/vacuum manifold. Perform three vacuum-refill cycles to replace the air in the flask with nitrogen gas.
- Add 138 ml anhydrous diethyl ether (Et2O, 0.200 M) to the reaction flask using an airtight syringe. Cool and stir the reaction mixture at 0 °C for 15 min.
- Prepare a solution of acetyl chloride (2.17 ml, 2.39 g, 30.4 mmol, 1.20 equiv) in anhydrous Et2O (138 ml, 0.220 M). Add the solution to the reaction mixture at 0 °C using a syringe pump at a rate of 10.0 ml/hr.
- At the end of the addition, filter the reaction mixture through a short pad of diatomaceous earth (i.e., Celite, 5 g) in 60 ml frit Buchner funnel using vacuum filtration. Wash the filter with EtOAc (20 ml x 3 times). Concentrate the filtrate in vacuo using a rotary evaporator.
- Purify the crude product with flash column chromatography35 eluting with hexanes:EtOAc (4:1 to 1:1 (v/v)) (Rf = 0.13, hexanes:EtOAc (4:1 (v/v)) to afford 5.31 g of methyl 4-(N-hydroxyacetamido)benzoate as a light yellow solid (25.4 mmol, 92% yield).
2. Synthesis of Methyl 4-(N-(trifluoromethoxy)acetamido)benzoate (2a)
- Add 2.00 g of methyl 4-(N-hydroxyacetamido) benzoate (1a) (9.56 mmol, 1.00 equiv), 311 mg of Cs2CO3 (0.956 mmol, 10.0 mol%), 3.63 g of Togni reagent II (11.5 mmol, 1.20 equiv), and a magnetic stir-bar into an oven-dried 250 ml round-bottom flask inside a glovebox (nitrogen atmosphere).
NOTE: This reaction can also be performed using Schlenk techniques outside the glovebox.
Caution: Pure Togni reagent II is impact and friction sensitive, open flames, sparks, and/or grinding should be avoided. Soft and polished tools should be used for manipulations. In addition, the reaction mixture should be stirred behind a safety shield.36 - Add 95.6 ml of dried and degassed chloroform (CHCl3, 0.100 M) to the reaction flask.
- Cap the flask with septum and stir the reaction mixture at 23 °C under N2 atmosphere either inside or outside of the glovebox for 16 hr.
- Filter the reaction mixture through a filter funnel to remove any solid residue. Concentrate the filtrate in vacuo using a rotary evaporator.
- Purify the crude product with flash column chromatography eluting with hexanes:dichloromethane (CH2Cl2) (7:3 to 0:1 (v/v)) (Rf = 0.44 (CH2Cl2) to afford 2.51 g of methyl 4-(N-(trifluoromethoxy)acetamido)benzoate (9.05 mmol, 95% yield).
NOTE: Togni reagent II is prepared according to the literature procedures37 and stored in the glovebox freezer at -35 °C to maintain its quality over a long period of time. This reaction is oxygen sensitive. Although all the reagents can be weighed out under ambient atmosphere at RT, removal of all oxygen from the reaction flask is critical. Dried and degassed CHCl3 is prepared by distilling it from CaH2 under nitrogen atmosphere followed by performing a three cycles of the freeze-pump-thaw procedure.
3. Synthesis of Methyl 4-Acetamido-3-(trifluoromethoxy)benzoate via OCF3-migration (3a)
- Add 2.51 g methyl 4-(N-(trifluoromethoxy)acetamido)benzoate (9.05 mmol, 1.0 equiv), a magnetic stir-bar, and 9.05 ml of MeNO2 (1.00 M) into a 50 ml pressure vessel. Cap the vessel with a screw cap.
- Stir the reaction mixture at 120 °C behind the safety shield for 20 hr.
Caution: Impure nitromethane is explosive, so the reaction mixture should be stirred behind the safety shield. - Cool the reaction mixture to RT.
- Transfer the reaction mixture to a 100 ml round-bottom flask.
- Concentrate the reaction mixture in vacuo using a rotary evaporator.
- Purify the crude product with flash column chromatography eluting with hexanes:EtOAc (9:1 to 7:3 (v/v)) (Rf = 0.51 hexanes:EtOAc (4:1 (v/v)) to afford 2.13 g of methyl 4-acetamido-3-(trifluoromethoxy)benzoate (7.69 mmol, 85%).
NOTE: This reaction can be carried out under ambient atmosphere. Nitrogen atmosphere is not required. A round-bottom flask equipped with a water condenser can be used as an alternative reaction apparatus.
4. Characterization of New Products
- Characterize all the new compounds by 1H, 13C NMR spectroscopy and high-resolution mass spectroscopy and use 19F NMR spectroscopy to characterize compounds containing fluorine atoms.34
Representative Results
Methyl 4-(N-hydroxyacetamido)benzoate (1a) was synthesized in 92% isolated yield through a two-step procedure (i.e., reducing methyl 4-nitrobenzoate with hydrazine using 5% Rh/C as a catalyst to form methyl 4-(N-hydroxyamino)benzoate, followed by acetyl protection of the resulting hydroxylamine). O-Trifluoromethylation of 1a with Togni reagent II in the presence of catalytic amount of cesium carbonate (Cs2CO3) in chloroform at RT afforded the desired 4-(N-(trifluoromethoxy)acetamido)benzoate (2a) in 95% isolated yield. This compound underwent thermally induced OCF3-migration in MeNO2 at 120 °C to give the desired methyl 4-acetamido-3-(trifluoromethoxy)benzoate (3a) in 85% isolated yield.
The 1H, 13C, and 19F NMR spectrum of the final product 3a are depicted in Figure 1, Figure 2, and Figure 3, respectively. A distinguish quartet peak at 120.6 ppm with a large coupling constant (258.9 Hz) in 13C NMR spectra corresponds to the CF3 carbon. When the OCF3-migration takes place, a sharp change in the 19F NMR from -65 ppm (2a) to -58.1ppm (3a) is observed. The detail characterization data of 3a is reported as follow: Rf = 0.51 (hexanes/EtOAc 4:1 (v/v)). NMR Spectroscopy: 1H NMR (700 MHz, CDCl3, 25 °C, δ): 8.56 (d, J = 8.6 Hz, 1H), 7.97 (d, J = 8.6 Hz, 1H), 7.93 (s, 1H), 7.56 (br. s, 1H), 3.92 (s, 3H), 2.27 (s, 3H). 13C NMR (175 MHz, CDCl3, 25 °C, δ): 168.5, 165.6, 137.2, 134.7, 129.3, 125.8, 121.5, 120.8, 120.6 (q, J = 258.9 Hz), 52.5, 25.2. 19F NMR (376 MHz, CDCl3, 25 °C, δ): -58.1 (s). Mass Spectrometry: HRMS (ESI-TOF) (m/z): calcd for C11H11NO4F3 ([M + H]+) 278.0640, found 278.0643.
This protocol is general and applicable to a wide array of aromatic compounds (Table 1). The reaction tolerates a broad spectrum of functional groups including ester (3a, 3d), ketone (3b), nitrile (3c), ethers (3e, 3m), halogens (3g – 3l), CF3 group (3m, 3n), amide (3o) and heterocycle substituent (3o). The halogen substituents, especially Br and I, are particularly useful because they provide synthetic handles for further functionalization. In addition, high levels of ortho– over para-selectivity are observed (3f, 3k – 3l). In the presence of two non-identical ortho positions, low levels of regiocontrol are obtained (3d, 3e, 3k, 3m). Furthermore, the reaction temperature for the OCF3-migration step depends on the electronic nature of arenes. Generally, more electron deficient arenes require higher reaction temperature.
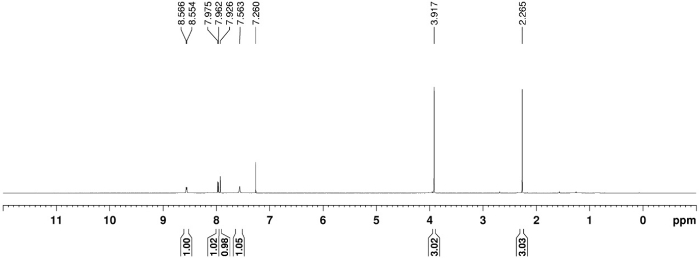
Figure 1. 1H NMR spectrum of 3a. Chemical shift and relative integration of characteristic protons are labeled. Please click here to view a larger version of this figure.
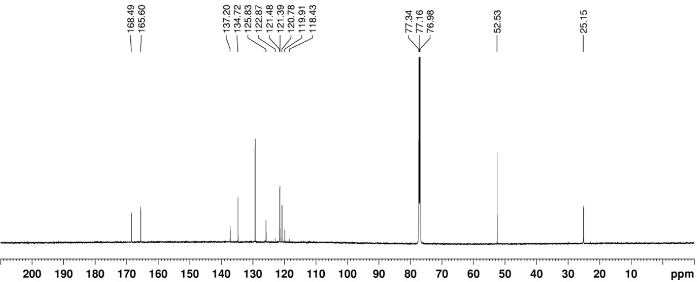
Figure 2. 13C NMR spectrum of 3a. Chemical shift of characteristic carbons is labeled. Please click here to view a larger version of this figure.
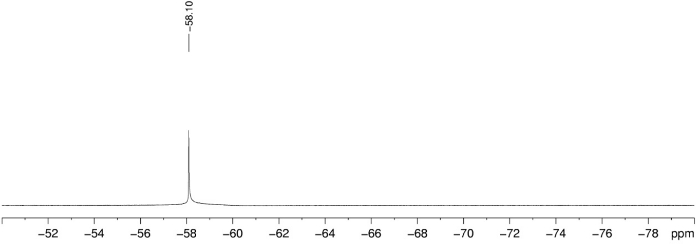
Figure 3. 19F NMR spectrum of 3a. Chemical shift of characteristic fluorine is labeled using trifluorotoluene (-63.3 ppm) as internal reference. Please click here to view a larger version of this figure.

Table 1. Selected examples of trifluoromethoxylation of arenes. Reaction time: 11-48 hr. Cited yields and isomeric ratios are for OCF3-migration step (from 2 to 3) and of isolated material by flash column chromatography. [a] 50 °C. [b] 120 °C. [c] 140 °C. [d] Less than 5% para-product was detected. THF = Tetrahydrofuran; AcCl = acetyl chloride. Please click here to view a larger version of this table.
Discussion
Due to the lack of a general and user-friendly procedure for the synthesis of trifluoromethoxylated arenes, many OCF3-containing aromatic compounds are extremely expensive.34 Our strategy displaces a broad functional group tolerance and provides an easy access to various trifluoromethoxylated arenes. These compounds could serve as valuable building blocks for the discovery and development of new pharmaceuticals, agrochemicals, and materials.
Hydrazine was used as a hydrogen source for the rhodium-catalyzed reduction of nitroarenes. Its quality is one of the keys in obtaining the reduction products in high yields. The reduction yields dropped when a few-month old hydrazine was used. To ensure the reproducibility, we transferred some of the hydrazine from a large commercial bottle to a smaller 20 ml vial and used it from the 20 ml vial. In addition, we stored it in the refrigerator (4 °C) to slow down the rate of decomposition. Moreover, slow addition of hydrazine is crucial in getting clean hydroxylamines in good yields.
The O-trifluoromethylation is a radical mediated process, so exclusion of oxygen from the reaction mixture is critical. Using un-degassed chloroform as the solvent or performing the reaction under ambient atmosphere resulted in lower yield. Our preliminary mechanistic studies shown that the OCF3-migration process involved thermally induced heterolytic cleavage of the N-OCF3 bond to generate a tight ion pair of nitrenium ion and trifluoromethoxide.34 Trifluoromethoxide attacks the ortho-position of the nitrenium ion followed by the tautomerization to afford the desired ortho-trifluoromethoxylated aniline derivatives. Formation of the nitrenium ion in electron deficient substrates is energetically disfavored and thus requires higher reaction temperature.
In summary, we reported a general and laboratory scale synthetic protocol for the regioselective synthesis of ortho-OCF3 aniline derivatives. This strategy has several unique features: (i) a wide range of functional groups and substitution patterns are tolerated; (ii) the operational simplicity of our protocol would render trifluoromethoxylation available to broader synthetic community; and (iii) the final products are novel and could be used as useful synthetic building blocks for life and materials science research. Some troubleshooting procedures are outlined here: (i) store the reduction product, aryl hydroxyl amine, in the freezer or immediately use it for the next step; (ii) monitor the reduction/protection reactions closely with TLC to avoid over reduction of nitroarenes or protection of N-hydroxyamines; (iii) exclusion of oxygen from the reaction mixtures is critical for the reduction of nitroarenes and O-trifluoromethylation; (iv) higher reaction temperature is needed for electron deficient arenes in the intramolecular OCF3-migration step.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We acknowledge generous start-up funds from the State University of New York at Stony Brook in support of this work. We also thank TOSOH F-Tech, Inc. for providing us TMSCF3 reagent for the synthesis of Togni reagent II.
Materials
| 5% Rhodium on carbon | Aspira Scientific | 300835 | 5% wt% dry loading |
| hydrazine monohydrate | Sigma-Alderich | 13696HMV | Reagent grade, 98% |
| Acetyl chloride | Alfa Aesar | 10176887 | 98% |
| Sodium bicarbonate | Fisher Scientific | 134826 | Chemical pure |
| Cesium carbonate | Alfa Aesar | 12887 | 99.9%, metals basis |
| Togni Reagent II | Prepared according to the literature procedure (ref 37). Caution: Pure Togni reagent II is impact and friction sensitive, treat it with great care (see ref. 36). | ||
| Tetrahydrofuran | BDH | BDH1149-4LG | Distilled from deep purple sodium benzophenone ketyl. |
| Diethyl Ether | Fisher Scientific | 148221 | Distilled from deep purple sodium benzophenone ketyl. |
| Chloroform | Fisher Scientific | 141739 | Dried over CaH2 and distilled |
| nitro methane | Alfa Aesar | J03z053 | Dried over CaSO4 and distilled |
| Silica gel | SILICYCLE | 60514 | 40-63 µm (230-400 mesh) |
| Cilite | EMD | 2012040674 | Not acid washed |
References
- Yagupolskii, L. M. Sintez proizvodnykh feniltriftormetilovogo efira. Dokl. Akad. Nauk SSSR. 105, 100-102 (1955).
- Booth, H. S., Burchfield, P. E. Fluorination of halogeno methyl ethers. I. Fluorination of trichlorodimethyl ether. J. Am. Chem. Soc. 57, 2070 (1935).
- McClinton, M. A., McClinton, D. A. Trifluoromethylations and related reactions in organic-chemistry. Tetrahedron. 48, 6555-6666 (1992).
- Hansch, C., Leo, A. . Substituent Constants for Correlation Analysis in Chemistry and Biology. , (1979).
- Leroux, F., Jeschke, P., Schlosser, M. Alpha-fluorinated ethers, thioethers, and amines: Anomerically biased species. Chem. Rev. 105, 827-856 (2005).
- Jeschke, P., Baston, E., Leroux, F. R. Alpha-fluorinated ethers as ‘exotic’ entity in medicinal chemistry. Mini-Rev. Med. Chem. 7, 1027-1034 (2007).
- Leroux, F. R., Manteau, B., Vors, J. P., Pazenok, S. Trifluoromethyl ethers – synthesis and properties of an unusual substituent. Beilstein J. Org. Chem. 4, (2008).
- Fantasia, S., Welch, J. M., Togni, A. Reactivity of a hypervalent iodine trifluoromethylating reagent toward THF: ring opening and formation of trifluoromethyl ethers. J. Org. Chem. 75, 1779-1782 (2010).
- Manteau, B., Pazenok, S., Vors, J. P., Leroux, F. R. New trends in the chemistry of alpha-fluorinated ethers, thioethers, amines and phosphines. J. Fluorine Chem. 131, 140-158 (2010).
- Landelle, G., Panossian, A., Leroux, F. R. Trifluoromethyl ethers and -thioethers as tools for medicinal chemistry and drug discovery. Curr. Top. Med. Chem. 14, 941-951 (2014).
- Liang, T., Neumann, C. N., Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 52, 8214-8264 (2013).
- Yarovenko, N. N., Vasileva, A. S. A new method for the introduction of trihalomethyl groups into organic molecules. Zh. Obshch. Khim. 28, 2502-2504 (1958).
- Yagupols, L., Troitskaya, V. I. Synthesis of phenyl trifluoromethyl ether derivatives. Zh. Obshch. Khim. 31, 915-924 (1961).
- Yagupolskii, L. M., Orda, V. V. Bis(triftormetoksi I triftormetilmerkapto)-proizvodnye benzola. Zh. Obshch. Khim. 34, 1979-1984 (1964).
- Louw, R., Franken, P. W. Selective side-chain chlorination of methoxybenzenes. Chem Ind-London. , 127-128 (1977).
- Feiring, A. E. Chemistry in hydrogen-fluoride. 7. Novel synthesis of aryl trifluoromethyl ethers. J. Org. Chem. 44, 2907-2910 (1979).
- Salome, J., Mauger, C., Brunet, S., Schanen, V. Synthesis conditions and activity of various Lewis acids for the fluorination of trichloromethoxy-benzene by HF in liquid phase. J. Fluorine Chem. 125, 1947-1950 (2004).
- Sheppard, W. A. Alpha-Fluorinated Ethers. I. Aryl Fluoroalkyl Ethers. J. Org. Chem. 29, 1-11 (1964).
- Kuroboshi, M., Suzuki, K., Hiyama, T. Oxidative desulfurization-fluorination of xanthates – a convenient synthesis of trifluoromethyl ethers and difluoro(methylthio)methyl ethers. Tetrahedron Lett. 33, 4173-4176 (1992).
- Kanie, K., Tanaka, Y., Suzuki, K., Kuroboshi, M., Hiyama, T. A convenient synthesis of trifluoromethyl ethers by oxidative desulfurization-fluorination of dithio carbonates. Bull. Chem. Soc. Jpn. 73, 471-484 (2000).
- Kuroboshi, M., Kanie, K., Hiyama, T. Oxidative desulfurization-fluorination: A facile entry to a wide variety of organofluorine compounds leading to novel liquid-crystalline materials. Adv. Synth. Catal. 343, 235-250 (2001).
- Umemoto, T. Electrophilic perfluoroalkylating agents. Chem. Rev. 96, 1757-1777 (1996).
- Umemoto, T., Adachi, K., Ishihara, S. CF3 oxonium salts, O-(trifluoromethyl)dibenzofuranium salts: in situ synthesis, properties, and application as a real CF3+ species reagent. J. Org. Chem. 72, 6905-6917 (2007).
- Stanek, K., Koller, R., Togni, A. Reactivity of a 10-I-3 hypervalent iodine trifluoromethylation reagent with phenols. J. Org. Chem. 73, 7678-7685 (2008).
- Koller, R., et al. Zinc-mediated formation of trifluoromethyl ethers from alcohols and hypervalent iodine trifluoromethylation reagents. Angew. Chem. Int. Ed. 48, 4332-4336 (2009).
- Trainor, G. L. The preparation of O-trifluoromethyl carbohydrates. J. Carbohydr. Chem. 4, 545-563 (1985).
- Nishida, M., Vij, A., Kirchmeier, R. L., Shreeve, J. M. Synthesis of polyfluoro aromatic ethers – a facile route using polyfluoroalkoxides generated from carbonyl and trimethysilyl compounds. Inorg. Chem. 34, 6085-6092 (1995).
- Kolomeitsev, A. A., Vorobyev, M., Gillandt, H. Versatile application of trifluoromethyl triflate. Tetrahedron Lett. 49, 449-454 (2008).
- Marrec, O., Billard, T., Vors, J. P., Pazenok, S., Langlois, B. R. A deeper insight into direct trifluoromethoxylation with trifluoromethyl triflate. J. Fluorine Chem. 131, 200-207 (2010).
- Marrec, O., Billard, T., Vors, J. P., Pazenok, S., Langlois, B. R. A new and direct trifluoromethoxylation of aliphatic substrates with 2,4-dinitro(trifluoromethoxy)benzene. Adv. Synth. Catal. 352, 2831-2837 (2010).
- Huang, C. H., Liang, T., Harada, S., Lee, E., Ritter, T. Silver-mediated trifluoromethoxylation of aryl stannanes and arylboronic acids. J. Am. Chem. Soc. 133, 13308-13310 (2011).
- Rozen, S. Selective fluorinations by reagents containing the OF group. Chem. Rev. 96, 1717-1736 (1996).
- Venturini, F., et al. Direct trifluoro-methoxylation of aromatics with perfluoro-methyl-hypofluorite. J. Fluorine Chem. 140, 43-48 (2012).
- Hojczyk, K. N., Feng, P., Zhan, C., Ngai, M. -. Y. Trifluoromethoxylation of arenes: synthesis of ortho-trifluoromethoxylated aniline derivatives by OCF3 migration. Angew. Chem. Int. Ed. 53, 14559-14563 (2014).
- Still, W. C., Kahn, M., Mitra, A. Rapid chromatographic technique for preparative separations with moderate Resolution. J. Org. Chem. 43, 2923-2925 (1978).
- Fiederling, N., Haller, J., Schramm, H. Notification about the Explosive Properties of Togni’s Reagent II and One of Its Precursors. Org. Process Res. Dev. 17, 318-319 (2013).
- Matousek, V., Pietrasiak, E., Schwenk, R., Togni, A. One-pot synthesis of hypervalent iodine reagents for electrophilic trifluoromethylation. J. Org. Chem. 78, 6763-6768 (2013).

