Formation of Human Periodontal Ligament Cell Spheroids on Chitosan Films
Summary
Here, we present protocols of culturing human periodontal ligament (PDL) cell spheroids by chitosan films. The culture of three-dimensional (3D) cellular spheroids provides an alternative to conventional tissue culture polystyrene (TCPS) culture system.
Abstract
Periodontal ligament (PDL) cells hold great promise for periodontal tissue regeneration. Conventionally, PDL cells are cultured on two-dimensional (2D) substrates such as tissue culture polystyrene (TCPS). However, characteristic changes of PDL cells have been observed during in vitro culture. This phenomenon is probably because the 2D TCPS differs from the in vivo three-dimensional (3D) microenvironment. Compared to cells cultured on 2D substrates, cells grown in a 3D microenvironment exhibit more similarities to in vivo cells. Therefore, 3D cell culture models provide a promising alternative for conventional 2D monolayer cell culture. To improve conventional PDL cell culture models, we have recently developed a 3D cell culture method, which is based on spheroid formation of PDL cells on chitosan films. Here, we present detailed cell spheroid culture protocols based on chitosan films. The 3D culture system of PDL cellular spheroids overcome some of the limitations related to conventional 2D monolayer cell culture, and thus may be suitable for producing PDL cells with an enhanced therapeutic efficacy for future periodontal tissue regeneration.
Introduction
Periodontitis, initialized principally by dental plaque1, is characterized by the damage of periodontal tissues including periodontal ligament (PDL), alveolar bone, and cementum. Current treatments for periodontitis are usually successful in preventing the progress of the active disease, but the regeneration of lost periodontal tissues remains a clinical challenge. Recently, important progress has been made in cell-based approaches for periodontal tissue regeneration to overcome the drawbacks of current treatments2,3,4.
Our previous systematic review revealed that PDL cells showed great potential for periodontal regeneration5. Conventionally, PDL cells are cultured on two-dimensional (2D) substrates such as tissue culture polystyrene (TCPS). However, characteristic changes of PDL cells have been observed during in vitro culture6. This phenomenon is probably because the 2D TCPS differs from the in vivo three-dimensional (3D) microenvironment7. Compared to cells cultured on 2D substrates, cells grown in a 3D microenvironment exhibit more similarities to in vivo cells8. Therefore, 3D cell culture models provide a promising alternative for conventional 2D monolayer cell culture.
Conventional 3D culture method is encapsulating cells in 3D biomaterials. Compared with cells encapsulated in 3D biomaterials, cellular spheroids mimic the in vivo situation more closely because spheroids are aggregates of cells growing free of foreign materials9,10,11,12. It is reported that cellular spheroids promoted MSC bioactivities via the preservation of extracellular matrix (ECM) components including fibronectin and laminin13. To improve conventional PDL cell culture models, we have recently developed a 3D PDL cell culture method, which is based on spheroid formation of PDL cells on chitosan films14. Spheroid formation increased the self-renewal and osteogenic differentiation capacities of PDL cells14. Here, we present detailed PDL cell spheroid culture protocols based on chitosan films. The 3D culture system of PDL cellular spheroids overcome some of the shortcomings related to conventional TCPS cell culture, and thus may be suitable for producing PDL cells with an enhanced therapeutic efficacy for future periodontal tissue regeneration.
Protocol
The study protocol was approved by the Ethics Committee of School and Hospital of Stomatology, Tongji University. All patients provided written informed consent.
1. PDL cell isolation
- Make proliferation medium for culture of PDL cells: α-MEM medium supplemented with 10% FCS and 100 U/mL pen/strep.
- Prepare a container with ice to transfer isolated third molars.
- Sterilize surgical instruments by using an autoclave.
- Extract normal human impacted third molars from adults (18-28 years of age) at the Dental Clinic of School and Hospital of Stomatology, Tongji University.
- Place third molars into the proliferation medium and transfer into the laboratory at 4 °C.
- Place the extracted tooth in a sterile 100 mm Petri dish, working within a biohazard laminar flow hood.
- Add 10 mL of PBS to wash the extracted tooth. Repeat the washing step twice.
- By using a sterile scalpel, gently separate the PDL from the root.
NOTE: PDL should be scraped from the middle third part of the root to avoid contaminations from gingival or apical tissues. - Mince PDL into 1-2-mm fragments with the help of a sterilized scalpel blade.
- Place PDL fragments in a T-25 culture flask, filled with 3-4 mL of proliferation medium.
- Culture the samples in an incubator at 37 °C in 5% CO2 with humidified air.
- Change the culture medium after outgrowth of PDL cells was observed. It usually takes 1-2 weeks for the outgrowth of PDL cells. Observe the outgrowth of PDL cells via an inverse phase contrast microscope.
- Change the culture medium twice per week thereafter.
- When PDL cells have reached confluence, sub-culture cells at ratio of 1:4 (passage 1).
- Observe cell growth daily and change the culture medium twice per week.
- Perform each subsequent passage at ratio of 1:4 after the cells achieve 80% confluence. Use third- to fifth-generation of PDL cells for cell seeding.
2. Preparation of chitosan films
- For the preparation of a 1% (v/v) acetic acid solution, add 5 mL of acetic acid to 495 mL of double-distilled water in a clean glass beaker.
- For the preparation of 1% w/v chitosan solution, weigh 5 g of chitosan powder (average molecular weight 400 kDa, 85% degree of acetylation) and add to 495 mL of 1% (v/v) acetic acid solution.
- Stir the prepared solution for 24 hours with stirring bar and magnetic stirring plate at 60 °C.
- Autoclave the prepared solution to ensure complete dissolution of chitosan.
NOTE: The experiment can be paused at this stage. - To form chitosan films, add 1% w/v chitosan solution into tissue culture polystyrene (TCPS) dishes at 0.25 mL/cm2. For example, add 0.5 mL of 1% w/v chitosan solution to one well of 24 –well plate.
- Dry TCPS dishes in an oven at 60 °C for 24 hours to form chitosan films.
- Prepare 0.5 N sodium hydroxide (NaOH). Stir 10 g of NaOH, a little at a time, into a large volume of double-distilled water by using the magnetic stir bar, and then dilute the solution to make 0.5 L.
NOTE: Add NaOH to water–do not add water to solid NaOH. Do not touch NaOH! It could cause chemical burns. - Neutralize chitosan films with 0.5 N NaOH. Add 0.5 mL of 0.5 N NaOH solution to one well of 24-well plate for 2 hours.
- Wash chitosan films three times with double-distilled water.
NOTE: The experiment can be paused at this stage. - Prior to cell seeding, sterilize the chitosan-coated 24-well plates in 70% alcohol overnight at room temperature.
- Rinse chitosan-coated plates three times with PBS at room temperature.
- Sterilize chitosan-coated plates by treatment under ultraviolet light overnight at room temperature.
3. Cell seeding
- Pre-warm proliferation medium PBS solution and trypsin/EDTA solution to 37 °C.
- Discard the supernatant from the T-75 cell culture flask.
- Wash confluent PDL cells with 10 mL of PBS solution.
- Discard the PBS solution.
- Add 1 mL of trypsin/EDTA solution to T-75 cell culture flask.
- Incubate cells with trypsin/EDTA solution for 3 min at 37 °C.
- Terminate the digestion process of trypsin by adding 3 mL of proliferation medium to T-75 cell culture flask.
- Transfer the prepared cell suspension into a 15 mL conical centrifuge tube.
- Centrifuge the suspension for 5 min at 300 x g and room temperature.
- Discard the supernatant from the 15 mL conical centrifuge tube and re-suspend the cell pellet in 500 µL of proliferation medium.
- Count PDL cells with a hemocytometer.
- Seed PDL cells at the densities of 0.5 x 104, 1 x 104, 3 x 104, and 6 x 104 cells/cm2.
- Culture PDL cells on chitosan films in an incubator at 37 °C in 5% CO2 with humidified air.
- Change the culture medium twice per week thereafter.
- On days 1, 2, 3, 4, and 5, observe the cell morphology daily via an inverse phase contrast microscope.
4. Cell survival
- After 1, 3, and 6 days of culture, determine the survival of cells using a commercial viability/cytotoxicity kit.
- In a 15 mL tube, prepare 2 mL of a fresh solution of 2 mM calcein-AM and 4 mM ethidium homodimer in PBS by vortexing for 5 s.
NOTE: Keep the process in the dark. - Remove the culture medium and wash spheroids in PBS.
- Incubate spheroids in of PBS containing 2 mM calcein-AM and 4 mM ethidium homodimer for 30 min at room temperature in the dark.
- Rinse spheroids twice in PBS. For spheroids from one well of 24-well plate, use 2 mL of PBS for each time.
- Observe the samples by using a fluorescence microscope using 10x or 20x magnification.
Representative Results
Using the present protocol, viable PDL cell spheroids were successfully formed. Figure 1 showed that suspended cells or spheroids instead of attached cells were mainly observed on chitosan films. For the seeding density of 0.5 x 104 cells/cm2, attached PDL cells were occasionally found on day 1 and 3, and PDL cell spheroids were rarely observed. On the contrary, for the seeding densities of 3 x 104 and 6 x 104 cells/cm2, various sizes of PDL cell spheroids were found since day 1. PDL cell spheroid formation was observed from all the seeding densities after 3 days. As shown in Figure 1, 3 x 104 cells/cm2 was the optimal PDL cell seeding density because the size of PDL cell spheroids was homogeneous at this cell seeding density. After 5 days of culture, larger spheroids were formed for all the cell seeding densities. Suspended PDL cells were rarely found on day 5.
The viability of PDL cells in spheroids was assessed after 1, 3 and 6 days. As shown in Figure 2, the majority of cells in spheroids were living cells on day 1, 3, and 6. While on day 6, the number of dead cells was increased in the central part.
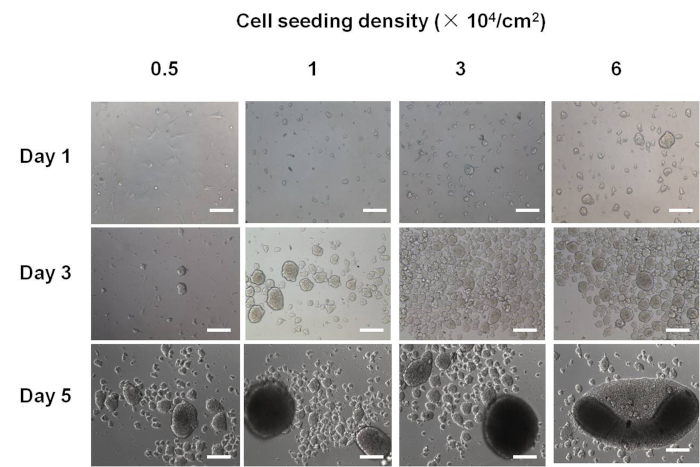
Figure 1. The morphology of PDL cellular spheroids.
For the seeding density of 0.5 x 104 cells/cm2, PDL cell spheroids were rarely observed on day 1 and 3. For the seeding densities of 3 x 104 and 6 x 104 cells/cm2, various sizes of PDL cell spheroids were found since day 1. PDL cell spheroid formation was observed from all the seeding densities after 3 days. After 5 days of culture, larger spheroids were formed for all the cell seeding densities. Suspended PDL cells were rarely found on day 5. Scale bar: 200 μm. This figure has been modified from Yan et al.14. Please click here to view a larger version of this figure.
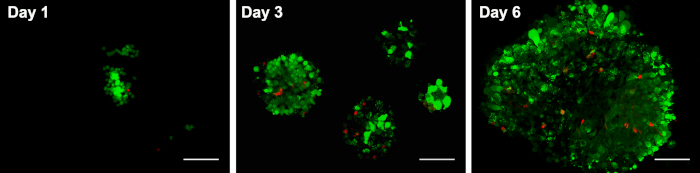
Figure 2. The viability of PDL cellular spheroids on chitosan films.
The viability of PDL cells in spheroids was assessed after 1, 3 and 6 days. As shown here, the majority of cells in spheroids were living cells on day 1, 3, and 6. While on day 6, the number of dead cells was increased in the central part. Scale bars: 200 μm. This figure has been modified from Yan et al.14. Please click here to view a larger version of this figure.
Discussion
The present study introduced a 3D cell culture system to overcome some limitations related to conventional 2D monolayer cell culture. According to the protocol, PDL cellular spheroids were successfully formed by culturing cells on chitosan films. Our previous study reported that spheroid formation increased the self-renewal and osteogenic differentiation capacities of PDL cells14. Instead of using an enzyme to harvest cells from TCPS, PDL cell spheroids could be harvested from chitosan films by simply pipetting the medium a few times14. Thus, ECM and intercellular junctions can be well preserved.
The critical steps of this protocol include: (1) making sure that the surgical instruments and chitosan films are sterilized for cell culture; (2) scraping the PDL from the middle third part of the root to avoid contaminations from gingival or apical tissues; and (3) requiring higher cell seeding densities (≥3 x 104 cells/cm2) for the successful spheroid formation of PDL cells.
However, one limitation for this method is that decreased proliferation of PDL cells was observed after spheroid formation14, which is similar to some previous studies that performed on adipose stromal cells10 and hepatocellular carcinoma cell line15. This is probably caused by the difference in the regulation of cyclin-dependent kinase inhibitors between spheroid and monolayer cells16. To benefit clinical application, further studies to promote the proliferation of cell spheroids are required.
Another drawback of the cellular spheroid is the inadequate diffusion in the core. Although PDL cells were mainly alive in spheroid culture, the number of dead cells was increased in the central part of spheroids on day 6. This phenomenon was probably because the diffusion of oxygen and nutrients is compromised in the spheroid core as PDL cell spheroids become larger17,18.
Other methods that reported to induce cellular spheroids include a non-adherent surface19, microwells20, spinner flasks21, micropatterned surfaces22,23, hanging drops24, and a forced-aggregation technique25. Compared to aforementioned methods, chitosan films are relatively cost-effective and easy to reproduce. Another advantage of this method is the antimicrobial properties of chitosan26, which is significantly beneficial for in vitro cell culture.
In summary, here we present detailed 3D cell spheroid culture protocols based on chitosan films. The culture of PDL cellular spheroids overcome some of the limitations related to conventional 2D TCPS cell culture, and thus may be suitable for producing PDL cells with an enhanced therapeutic efficacy for future periodontal tissue regeneration.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was sponsored by National Natural Science Foundation of China (NSFC 81700978), Fundamental Research Funds for the Central Universities (1504219050), Natural Science Foundation of Shanghai (17ZR1432800), and Shanghai Medical Exploration Project (17411972600).
Materials
| α-MEM | Gibco | 11900-073 | |
| acetic acid | Sigma-Aldrich | 64197 | |
| Cell culture flask 25 cm2 | Corning | 430639 | |
| Cell culture flask 75 cm2 | Corning | 430641 | |
| Chitosan | Heppe Medical Chitosan GmbH | / | molecular weight 500 kDa, degree of deacetylation 85% |
| FCS | Gibco | 26140-079 | |
| Live/Dead Viability/Cytotoxicity Kit | Molecular Probes | L3224 | |
| NaOH | Sigma-Aldrich | 1310732 | |
| PBS | KeyGen Biotech | KGB5001 | |
| pen/strep | Gibco | 15140-122 | |
| Trypsin/EDTA | KeyGen Biotech | KGM25200 | |
| 15 mL conical centrifuge tube | Corning | 430790 | |
| 24-well plate | Corning | 3524 |
References
- Albandar, J. M. Epidemiology and risk factors of periodontal diseases. Dental Clinics of North America. 49 (3), 517-532 (2005).
- Bartold, P. M., McCulloch, C. A., Narayanan, A. S., Pitaru, S. Tissue engineering: a new paradigm for periodontal regeneration based on molecular and cell biology. Periodontology 2000. 24, 253-269 (2000).
- Chen, F. M., Jin, Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Engineering Part B Review. 16 (2), 219-255 (2010).
- Yu, N., et al. Enhanced periodontal tissue regeneration by periodontal cell implantation. Journal of Clinical Periodontology. 40 (7), 698-706 (2013).
- Yan, X. Z., Yang, F., Jansen, J. A., de Vries, R. B., van den Beucken, J. J. Cell-Based Approaches in Periodontal Regeneration: A Systematic Review and Meta-Analysis of Periodontal Defect Models in Animal Experimental Work. Tissue Engineering Part B Review. 21 (5), 411-426 (2015).
- Itaya, T., et al. Characteristic changes of periodontal ligament-derived cells during passage. Journal of Periodontal Research. 44 (4), 425-433 (2009).
- Zhang, J., Li, B., Wang, J. H. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 32 (29), 6972-6981 (2011).
- Elliott, N. T., Yuan, F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. Journal of Pharmaceutical Sciences. 100 (1), 59-74 (2011).
- Fennema, E., Rivron, N., Rouwkema, J., van Blitterswijk, C., de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends in Biotechnology. 31 (2), 108-115 (2013).
- Cheng, N. C., Wang, S., Young, T. H. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 33 (6), 1748-1758 (2012).
- Yeh, Y. C., et al. Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials. 31 (25), 6444-6453 (2010).
- Kabiri, M., et al. 3D mesenchymal stem/stromal cell osteogenesis and autocrine signalling. Biochemical and Biophysical Research Communications. 419 (2), 142-147 (2012).
- Lee, J. H., Han, Y. S., Lee, S. H. Long-Duration Three-Dimensional Spheroid Culture Promotes Angiogenic. Activities of Adipose-Derived Mesenchymal Stem Cells. Biomolecules & therapeutics. 24 (3), 260-267 (2016).
- Yan, X. Z., van den Beucken, J., Yuan, C., Jansen, J. A., Yang, F. Spheroid formation and stemness preservation of human periodontal ligament cells on chitosan films. Oral Diseases. 24 (6), 1083-1092 (2018).
- Meli, L., Jordan, E. T., Clark, D. S., Linhardt, R. J., Dordick, J. S. Influence of a three-dimensional, microarray environment on human Cell culture in drug screening systems. Biomaterials. , (2012).
- LaRue, K. E., Khalil, M., Freyer, J. P. Microenvironmental regulation of proliferation in multicellular spheroids is mediated through differential expression of cyclin-dependent kinase inhibitors. Cancer Research. 64 (5), 1621-1631 (2004).
- Tsai, A. C., Liu, Y., Yuan, X., Ma, T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue Engineering Part A. 21 (9-10), 1705-1719 (2015).
- Cesarz, Z., Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells International. 2016, 9176357 (2016).
- Tong, J. Z., Sarrazin, S., Cassio, D., Gauthier, F., Alvarez, F. Application of spheroid culture to human hepatocytes and maintenance of their differentiation. Biology of the Cell. 81 (1), 77-81 (1994).
- Lee, W. Y., et al. The use of injectable spherically symmetric cell aggregates self-assembled in a thermo-responsive hydrogel for enhanced cell transplantation. Biomaterials. 30 (29), 5505-5513 (2009).
- Frith, J. E., Thomson, B., Genever, P. G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Engineering Part C Methods. 16 (4), 735-749 (2010).
- Wang, W., et al. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 30 (14), 2705-2715 (2009).
- Miyagawa, Y., et al. A microfabricated scaffold induces the spheroid formation of human bone marrow-derived mesenchymal progenitor cells and promotes efficient adipogenic differentiation. Tissue Engineering Part A. 17 (3-4), 513-521 (2011).
- Bartosh, T. J., et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proceedings of the National Academy of Sciences of the United States of America. 107 (31), 13724-13729 (2010).
- Baraniak, P. R., McDevitt, T. C. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell and Tissue Research. 347 (3), 701-711 (2012).
- Rabea, E. I., Badawy, M. E., Stevens, C. V., Smagghe, G., Steurbaut, W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 4 (6), 1457-1465 (2003).

