Combined Infusion and Stimulation with Fast-Scan Cyclic Voltammetry (CIS-FSCV) to Assess Ventral Tegmental Area Receptor Regulation of Phasic Dopamine
Summary
The goal of this protocol is to directly manipulate ventral tegmental area receptors to study their contribution to subsecond dopamine release.
Abstract
Phasic dopamine (DA) release from the ventral tegmental area (VTA) to the nucleus accumbens plays a pivotal role in reward processing and reinforcement learning. Understanding how the diverse neuronal inputs into the VTA control phasic DA release can provide a better picture of the circuitry that controls reward processing and reinforcement learning. Here, we describe a method that combines intra-VTA cannula infusions of pharmacological agonists and antagonists with stimulation-evoked phasic DA release (combined infusion and stimulation, or CIS) as measured by in vivo fast-scan cyclic voltammetry (FSCV). Using CIS-FSCV in anesthetized rats, a phasic DA response can be evoked by electrically stimulating the VTA with a bipolar electrode fitted with a cannula while recording in the nucleus accumbens core. Pharmacological agonists or antagonists can be infused directly at the stimulation site to investigate specific VTA receptors' roles in driving phasic DA release. A major benefit of CIS-FSCV is that VTA receptor function can be studied in vivo, building on in vitro studies.
Introduction
Phasic dopamine (DA) release from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) plays a vital role in reward-related behaviors. VTA DA neurons switch from a tonic-like firing (3-8 Hz) to a burst-like firing (>14 Hz)1, which produces phasic DA release in the NAc. The VTA expresses a variety of somatodendritic receptors that are well-positioned to control the switch from tonic to burst-firing2,3,4,5. Identifying which of these receptors, and their respective inputs, control phasic DA release will deepen our understanding of how the reward-related circuitry is organized. The purpose of the methodology described here, combined infusion and stimulation with fast-scan cyclic voltammetry (CIS-FSCV), is to quickly and robustly assess the functionality of VTA receptors in driving phasic DA release.
The term combined infusion and stimulation (CIS) refers to pharmacologically manipulating receptors on a group of neurons (here the VTA) and stimulating those neurons to study the receptor's function. In the anesthetized rat, we electrically stimulate the VTA to evoke a large phasic DA signal (1-2 µM) in the NAc core, as measured by fast-scan cyclic voltammetry (FSCV). Infusions of pharmacological drugs (i.e., receptor agonists/antagonists) at the stimulation site can be used to measure the function of VTA receptors by observing the subsequent change in evoked phasic DA release. FSCV is an electrochemical approach that enjoys both high spatial (50-100 µm) and temporal (10 Hz) resolution, and is well-suited to measure reward-related, phasic DA events6,7. This resolution is finer than other in vivo neurochemical measurements, such as microdialysis. Thus, together, CIS-FSCV is well-suited to assess VTA receptor regulation of phasic dopamine release.
One common way to investigate VTA receptor function is by using a combination of electrophysiological approaches that address how those receptors alter the firing rate of neurons1,8. These studies are highly valuable in understanding what receptors are involved in driving DA firing upon activation. However, these studies can only suggest what might happen downstream at the axon terminal (i.e., release of a neurotransmitter). CIS-FSCV builds on these electrophysiological studies by answering how the output of VTA burst-firing, phasic DA release, is regulated by receptors located on VTA dendrites and cell bodies. Thus, CIS-FSCV is well-suited to build on these electrophysiology studies. As an example, nicotinic receptor activation can induce burst-firing in the VTA9, and CIS-FSCV in the anesthetized rat was used to show that nicotinic acetylcholine receptor (nAChR) activation in the VTA also controls phasic DA release in the NAc10,11.
Mechanistic examination of phasic DA regulation is also commonly studied using slice preparations alongside with bath application of drugs. These studies often focus on the presynaptic regulation of phasic DA release from dopamine terminals, as the cell bodies are often removed from the slice12. These preparations are valuable for studying presynaptic receptor effects on dopamine terminals, whereas CIS-FSCV is better suited to study somatodendritic receptor effects on dopamine neurons, as well as presynaptic inputs to the VTA. This distinction is important, because somatodendritic receptor activation in the VTA may have a different effect than NAc presynaptic receptor activation. Indeed, blocking dopaminergic presynaptic nAChRs in the NAc can elevate phasic dopamine release during burst-firing13, whereas the opposite is true at VTA somatodendritc nAChRs10,11.
CIS-FSCV is an ideal approach for studying the ability of VTA receptors to regulate phasic DA release. Importantly, this approach can be performed in an intact rat, either anesthetized or free moving. This approach is suitable for acute studies, to study receptor function in its baseline state10,14 as well as long-term studies that can assess functional changes in a receptor after drug exposure or behavioral manipulation11,15.
Protocol
All experiments were conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by both Elizabethtown College and Yale University Institutional Animal Care and Use Committee (IACUC). This protocol is specific to the anesthetized rat preparation of utilizing CIS-FSCV.
1. Presurgical preparations
- Electrode solution preparation
- To make the electrode backfill solution, prepare a solution of 4 M potassium acetate with 140 mM potassium chloride16.
- Electrode preparation
- Using vacuum suction, insert a T-650 carbon fiber (7 µm in diameter) into a borosilicate glass capillary (length = 100 mm, diameter = 1.0 mm, inside diameter = 0.5 mm).
- Once the carbon fiber has been placed inside the glass capillary, place the glass capillary into a vertical electrode puller, with the heat element roughly in the middle of the capillary. Set the heater to 55 with the magnet turned off.
- After the capillary is pulled, carefully raise the upper capillary holder so that the tip of the electrode is not surrounded by the heating element.
- Using sharp scissors, cut the carbon fiber that is still connecting the two pieces of the capillary. This will result in two separate carbon fiber microelectrodes.
- Under a light microscope, carefully cut the exposed carbon fiber with a sharp scalpel, so that the carbon fiber extends approximately 75-100 µm beyond the end of the glass.
- Using a light microscope, ensure that the electrode is free of cracks along the capillary. Also ensure that the seal, where the carbon fiber exits the capillary, is difficult to notice and free from cracks.
NOTE: A good seal will help reduce noise during recordings. See published studies17,18,19 for a more detailed protocol.
- Reference electrode fabrication
- Solder a gold pin to a 5 cm silver wire.
- Attach the anode to a metal paper clip or other conductor, the cathode to a pin, and apply a voltage (~2 V) while the paper clip and silver wire is submerged in 0.1 M HCl.
- Cease the voltage once a white coating (AgCl) appears on the silver wire.
- Preparing electrode for implantation
- Solder a gold pin to a thin insulated wire (~10 cm in length, <0.50 mm diameter).
- Remove ~5 cm of insulation from the wire opposite to the gold pin.
- Fill the electrode approximately halfway with electrode solution.
- Insert insulated wire into the electrode.
NOTE: The wire should make contact with the carbon fiber inside the electrode.
2. Electrode implantations
- Give adult, male, Sprague Dawley rats (250−450 g) an intraperitoneal injection (1.5 g/kg or 1 mL/kg volume) of 0.5 g/mL urethane dissolved in sterile saline. Start with an initial urethane dose of 1.0−1.2 g/kg. If the animal is still responsive to the noxious stimulus test (tail pinch) 20 min after urethane administration, administer an additional 0.3−0.5 g/kg urethane for a 1.5 g/kg total dose.
NOTE: For preparation of the 0.5 g/mL urethane solution, add 10 g of urethane to 10 g (~10 mL) of saline. Urethane is a carcinogen and must be handled with care. Urethane is an important anesthetic, as it does not alter levels of dopamine, as do other anesthetics such as ketamine/ xylazine and chloral hydrate20,21. - Once the animal is deeply anesthetized and is not responsive to noxious stimuli (e.g., toe pinch), place it in the stereotaxic frame. Apply ophthalmic lubricant to each eye of the rat.
NOTE: This is a non-survival surgery, but good aseptic technique is encouraged. - Clean the rat's scalp using a two-stage scrub (i.e., an iodopovidone scrub followed by a 70% ethanol scrub; perform with a 3 cycle repetition).
- Cut away the scalp tissue using sterilized needle nose tweezers and surgical scissors. Remove a significant amount of tissue to make room for the various implantations outlined below.
- Gently clean the skull surface using sterilized cotton tip applicators. Then apply 2−3 drops of 3% hydrogen peroxide to help identify the lambda and bregma.
- Using a stereotaxic or hand drill (1.0 mm, ~20,000 rpm), drill a 1.5 mm diameter hole 2.5 mm anterior to the bregma and 3.5 mm lateral to the bregma. Partially (about halfway, until it is firmly in place) implant a screw (1.59 mm O.D., 3.2 mm long) in this hole. It is recommended to use sterile saline to irrigate while drilling to prevent thermal injury.
- For the reference electrode, drill a 1.0 mm diameter hole 1.5 mm anterior and 3.5 mm lateral to the bregma, in the left hemisphere.
- By hand, insert ~2 mm of reference wire into this hole, while wrapping the reference wire around and under the head of the implanted screw.
- Fully implant the screw, pinning down the reference electrode in place.
- In the right hemisphere, drill a 1.5 mm diameter hole 1.2 mm anterior and 1.4 mm lateral to the bregma.
- Gently remove the dura using tweezers.
- For the stimulating electrode, drill a square hole (2 mm anterior-posterior, 5 mm medial-lateral) centered at 5.2 mm posterior and 1.0 mm lateral to the bregma.
- Using the stereotactic arm bars, lower the bipolar stimulating electrode/guide cannula 5 mm below the dura. In case of bleeding during the implantation of the electrode, use sterile cotton swabs and gauze to minimize bleeding.
NOTE: The bipolar stimulating electrode used in this method is prefitted with a guide cannula (Table of Materials). The internal cannula used with this item should be flushed with the prongs on the bipolar stimulating electrode when fully inserted into the guide cannula. This will allow the internal cannula to sit directly in between the two prongs of the stimulator, which sit about 1 mm apart. A similar protocol is described elsewhere14. - Using the stereotactic arm bars, lower the carbon fiber microelectrode 4 mm below the dura. This location is at the most dorsal portion of the striatum.
- Connect the reference wire and carbon fiber to a potentiostat.
- Apply a triangular wave form (-0.4−1.3 V, 400 V/s) for 15 min at 60 Hz, and again for 10 min at 10 Hz.
NOTE: Typically, when applying waveforms to carbon fiber microelectrodes in the brain, oxide groups are added to the surface of the carbon fiber. Equilibrium of this reaction must be reached prior to recording; otherwise significant drift will occur19. Cycling the electrode at higher frequencies (60 Hz) allows the carbon fiber to achieve equilibrium faster.
3. Optimizing carbon fiber and stimulating electrode/guide cannula locations
- Set the stimulator to produce a bipolar electrical waveform, with a frequency of 60 Hz, 24 pulses, 300 µA current, and pulse width of 2 ms/phase.
- Gently lower the stimulator in increments of 0.2 mm from 5 mm to 7.8 mm below the dura. At each increment, stimulate the VTA.
NOTE: At more dorsal depths (5−6 mm), stimulation of the brain will typically (~80% of the time) cause the whiskers of the rat to twitch. At further depths, the whiskers will cease twitching, which occurs between 7.5−8.2 mm below the dura. When the whiskers cease twitching, the stimulating electrode will be near or at the VTA. This will not occur in every rat, and lack of whisker twitching should not be taken as a sign that the bipolar stimulating electrode/infusion cannula is misplaced. Whisker twitching may not occur for all anesthetics (e.g., isoflurane). - Continue to lower the bipolar stimulating electrode/guide cannula until a stimulation produces phasic DA release at the carbon fiber microelectrode (currently in the dorsal striatum).
NOTE: DA release in the dorsal striatum will not always occur if the bipolar electrode is implanted in the VTA, but observation of DA release in the dorsal striatum upon VTA stimulation is usually a good sign that a good signal will be observed in the NAc core. - Lower the carbon fiber microelectrode until it is at least 6.0 mm below the dura. This is the most dorsal part of the NAc core.
- Stimulate the VTA and record the peak amplitude of the DA peak.
- Lower or raise the carbon fiber microelectrode at the site that produces the greatest DA release.
- Ensure that the peak of the DA response is a clear oxidation peak at 0.6 V and a reduction peak at -0.2 V. These peaks are indicative of DA.
4. Combination infusion and stimulation FSCV recording
NOTE: Figure 1 shows the timeline for recording before and after VTA microinfusion.
- Once the carbon fiber and stimulating electrode/guide cannula location has been optimized, stimulate for ~20−30 min.
NOTE: Under the current stimulation parameters, do not stimulate any more than once every 3 min, to allow for vesicular reloading22. - After achieving a stable baseline (<20% variation over five stimulations), gently lower the internal cannula by hand into the guide cannula that is prefitted into the bipolar stimulator.
- Take an additional 2−3 baseline recordings to ensure that the cannula insertion itself did not cause a change in the evoked signal. In some cases, insertion and removal of the internal cannula can cause damage to the VTA. If the signal drastically changes over this baseline period (>20%), then take an additional 3−4 recordings until the baseline restabilizes.
- Using a syringe pump and microsyringe, infuse 0.5 µL of solution (e.g., 0.9% saline, N-methyl-D-aspartate [NMDA], (2R)-amino-5-phosphonovaleric acid [AP5]) into the VTA over a 2 min period.
- Postinfusion, leave the internal cannula for at least 1 min prior to removal.
NOTE: Some drugs may require leaving the internal cannula for a longer time based on the drug kinetics, and removal of the internal cannula may cause the drug to travel back up through the internal cannula. If there is concern, one could leave the internal cannula in the guide cannula during the entirety of the recording. Otherwise, recording can begin after this 1 min interval. - Continue recording every 3 min to measure postinfusion effects.
NOTE: If infusing a control solution, and no effect is observed, it is possible to infuse a second time10. If there is altered DA release caused by inserting the internal cannula or saline infusion, the signal typically recovers to baseline within 30 min.
5. Histological verification of electrode placement
- At the end of the experiment, create a small lesion at the recording site using the carbon fiber microelectrode.
- If the electrode must be preserved for postexperiment calibration, then use a tungsten wire placed in a glass capillary protruding ~100 µm beyond the capillary tip. In this case, raise the electrode from the brain, replace the recording electrode with the tungsten electrode, and lower it to the same dorsoventral coordinate.
NOTE: The carbon fiber may be used to lesion the brain as well and will provide a more accurate representation of the location of the recording site; however, the experimenter will lose the ability to calibrate these electrodes.
- If the electrode must be preserved for postexperiment calibration, then use a tungsten wire placed in a glass capillary protruding ~100 µm beyond the capillary tip. In this case, raise the electrode from the brain, replace the recording electrode with the tungsten electrode, and lower it to the same dorsoventral coordinate.
- To lesion the recording site, apply voltage using a power supply. Start at 1 V and increase by 1 V every 10 s until 10 V is reached.
- Euthanize the animal using a lethal intraperitoneal injection of pentobarbital (150 mg/kg).
- Perfuse the rat using a 4% formalin solution.
- Remove the head from the rat using a sharpened guillotine.
- Using rongeurs, remove the connective tissue and skull surrounding the brain, and gently dislodge the brain from any remaining tissue.
- Store the brain in 4% formalin for 1 day and then transfer it to 30% sucrose.
NOTE: Perfusion with 4% formalin is not necessary to see the lesion site, although as a best practice it will improve the reconstruction of the lesion site. - Create 30 µm slices of the brain using a cryostat.
- Mount the slices on slides and cover with a cover slip.
- Denote the location of the carbon fiber microelectrode lesion and bipolar stimulator/infusion cannula location using a light microscope.
Representative Results
CIS-FSCV was used to study the function of VTA N-methyl-D-aspartate receptors (NMDAR), nicotinic acetylcholine receptors (nAChRs), and muscarinic acetylcholine receptors (mAChRs) in driving phasic DA release in the NAc core. Figure 2 shows representative data for a negative control, infusion of 0.9% saline, before (baseline) and 9 min postinfusion (saline). Figure 2 shows a color plot with potential on the y-axis, time on the x-axis, and current (represented as false color) on the z-axis, current versus time traces (IvT), as well as a cyclic voltammogram taken at the peak evoked response to demonstrate that the analyte measured corresponds to DA. As expected, saline infusion did not alter the stimulated phasic DA release.
To demonstrate that CIS-FSCV can produce bidirectional effects when using agonists and antagonists, we compared the effects of infusion of the NMDAR agonist, NMDA (500 ng; Figure 3A) to the NMDAR antagonist, AP5 (1 µg; Figure 3B). Infusion of NMDA produced a robust increase in stimulated phasic DA release (Figure 3A, 9 min after infusion) while the NMDAR competitive antagonist, AP5 (1 µg), produced a robust decrease (Figure 3B, 9 min after infusion). To demonstrate the utility of CIS-FSCV using antagonists that target different classes of acetylcholine receptors, we compared the effects of infusion of the nonselective, noncompetitive nAChR antagonist mecamylamine (3 µg; Figure 4A) and the nonselective, competitive mAChR antagonist scopolamine (67 µg; Figure 4B). Both drugs produced robust decreases in stimulated phasic DA release (Figure 4, 9 min postinfusion). A summary of the results from Figure 2, Figure 3, and Figure 4 are replotted in Figure 5, where the baseline period is averaged over five stimulations and the drug period is displayed as a percentage of the baseline average.
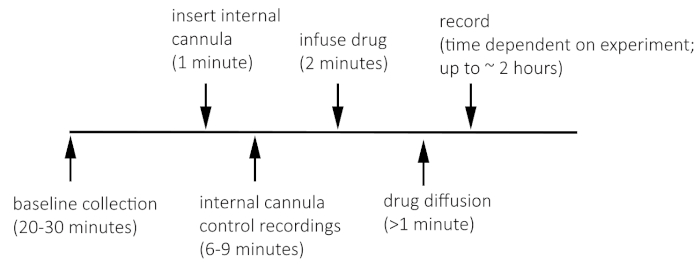
Figure 1: Timeline for recording before and after VTA microinfusion. Please click here to view a larger version of this figure.
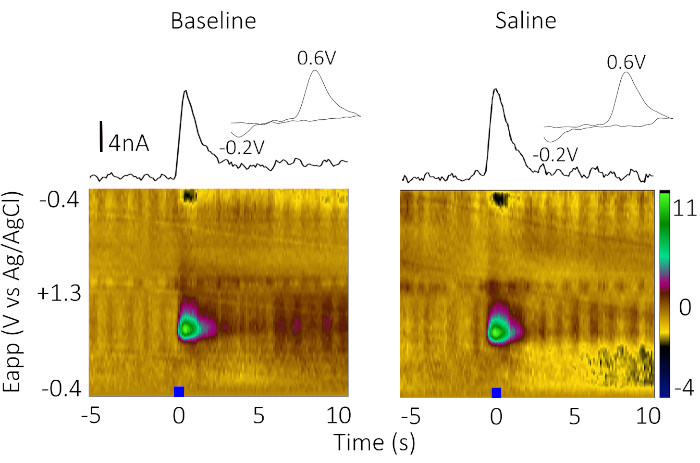
Figure 2: Representative color and IvT plots of baseline (left) and saline (vehicle) infusion (right) on stimulated phasic DA release in the NAc core in a single male Sprague Dawley rat. Blue bar represents stimulation. The baseline recording occurs at t = 0, before the internal cannula was placed in the guide cannula in the bipolar stimulator. The saline recording was taken 9 min (t = 9) postinfusion. Cyclic voltammogram insets correspond to the peak of the IvT plots, showing a peak oxidation at 0.6 V and peak reduction at -0.2 V, indicative of DA. No change in stimulated evoked release should be observed after saline VTA infusion. Please click here to view a larger version of this figure.
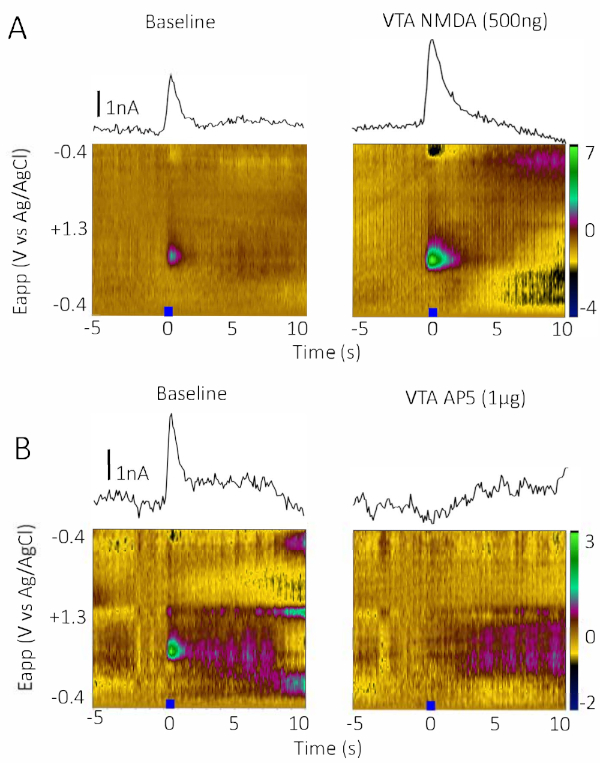
Figure 3: Effects of infusion of the NMDAR agonist (NMDA) and antagonist (AP5). (A) Representative color and IvT plots of baseline (left) and 500 ng of the NMDAR agonist infusion (right) on stimulated phasic DA release in the NAc core in a single male Sprague Dawley rat. NMDA infusion increased stimulated phasic DA release (recording taken 9 min postinfusion). (B) Representative color and IvT plots of baseline (left) and 1 µg of the NMDAR antagonist (2R)-amino-5-phosphonovaleric acid (AP5) infusion (right) on stimulated phasic DA release in the NAc core in a single male Sprague Dawley rat. AP5 infusion reduced stimulated phasic DA release (recording taken 9 min postinfusion). The baseline recordings occurred at t = 0, before the internal cannula was placed in the guide cannula in the bipolar stimulator. Please click here to view a larger version of this figure.

Figure 4: Effects of infusion of mecamylamine and scopolamine. (A) Representative color and IvT plots of baseline (left) and 3 µg of the nonselective nicotinic acetylcholine receptor antagonist mecamylamine (MEC) infusion (right) on stimulated phasic DA release in the NAc core in a single male Sprague Dawley rat. (B) Representative color and IvT plots of baseline (left) and 67 µg of the nonselective muscarinic acetylcholine receptor antagonist scopolamine (SCOP) infusion (right) on stimulated phasic DA release in the NAc core in a single male Sprague Dawley rat. The baseline recording occurred at t = 0, before the internal cannula was placed in the guide cannula in the bipolar stimulator. MEC and SCOP recordings were taken 9 min postinfusion. Please click here to view a larger version of this figure.
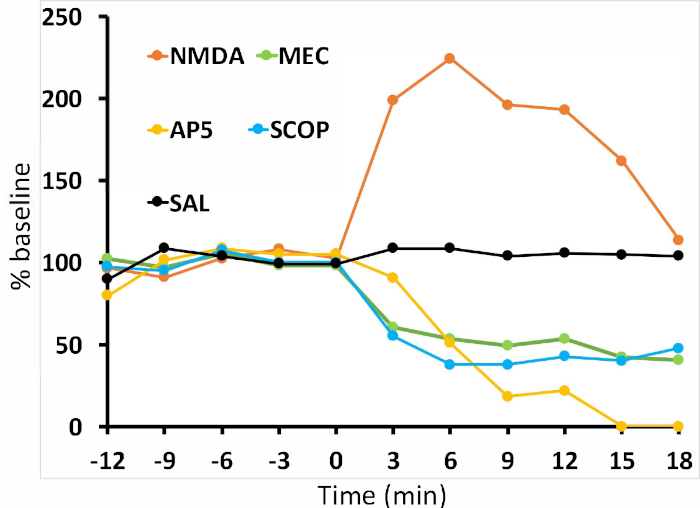
Figure 5: Data summary showing drug effects over time. Pre-infusion period (baseline) was averaged over five stimulations, and postinfusion period (starting at t = 3) is presented as a percentage of baseline. At 9 min postinfusion, we observed that the evoked DA signal was 103% of baseline after saline infusion, 196% after NMDA infusion, 18% after AP5 infusion, 49% after MEC infusion, and 43% after SCOP infusion. n = 1 per condition. Please click here to view a larger version of this figure.
Discussion
CIS-FSCV provides a unique opportunity to investigate VTA receptor mechanisms underlying phasic DA release. There are two critical steps in order to ensure a proper recording. First, a stable baseline recording must be achieved, with little drift in the evoked DA signal. An important way to increase the likelihood of establishing a stable recording is to ensure that the electrode has had plenty of time to cycle at both 60 Hz and 10 Hz (typically 15 min at 60 Hz, and 10 min at 10 Hz). As the carbon fiber is being cycled, the carbon fiber itself oxidizes, and becomes etched, decreasing the surface area but producing new surface for dopamine adsorption23. This can lead to an increase in sensitivity to DA. Thus, one might see a slight increase in stimulated dopamine release over time in the experiment due to this increased etching, rather than any pharmacological manipulation. Additionally, beginning a recording within 90 to 120 min of initial anesthesia will increase the likelihood of a stable recording over long periods of time. As such, as the rat nears death from anesthesia, it is typical for the evoked DA release to decrease slowly.
The second critical step in this procedure is to ensure that the infusion cannula is gently inserted into the bipolar stimulating electrode. The stereotaxic armbars can move if too much pressure is placed while inserting the internal cannula, and as a result, the dopamine signal might artificially increase or decrease, as the stimulation site may be different. If there is a significant change in evoked signal after cannula insertion, a new baseline period should be established. Moreover, if there is altered DA release caused by inserting the internal cannula or vehicle infusion, the signal typically recovers to baseline within 30 min. Should there be extensive alterations in DA release to vehicle infusion, the infusion rate or volume could be reduced. Investigators may also perform an additional recording after inserting the internal cannula before the infusion to assess whether insertion of the cannula itself can alter release. Relatedly, it is important to verify that the infusion occurred, and that there is no blockade of the internal cannula. One way to do this is to make a small bubble in the infusion tubing, and mark this with a pen or marker. The bubble should be further away from the marker after infusion. Another way to ensure that the infusion occurred properly is to turn on the infusion pump after the internal cannula has been removed from the brain, and if there is still solution forming at the tip, then a successful infusion likely has occurred.
CIS-FSCV can be adapted to study VTA receptors in both behaviorally naive and trained animals to study changes in receptor function over time11. CIS-FSCV can also be modified to measure 5-HT and norepinephrine (NE)24,25. CIS-FSCV is also highly suitable for awake, behavior experiments and can be integrated with optogenetic approaches26,27. It is important to note that electrically evoked release events are distinct from transient release events often observed in free-moving studies, and less often in the anesthetized preparation. Transient release events, for example, may not be necessarily be driven by direct depolarization of dopamine neurons unlike electrically evoked release events28. As such, phasic neural activity might be dissociable from the dopamine release events detected via FSCV. Moreover, optically evoked DA release has been shown to differ from electrically evoked DA release events. A recent comparison between optically and electrically evoked stimulation has revealed that electrically evoked stimulation produces multisynaptic regulation of phasic DA release, whereas optically evoked stimulation can limit stimulation to more specific circuits29.
Some recent approaches have employed optogenetic and fluorescent methods to investigate the circuitry underlying rapid dopamine dynamics in vivo30. For example, recent work by Sun and colleagues showed that optogenetic stimulation of dopamine neurons in the substantia nigra produces rapid elevations of DA in the striatum, as measured via expression of G-protein coupled receptor-activation-based DA (GRABDA) sensors30. Combined optogenetic and fluorescence approaches could be used to stimulate or inhibit specific afferent inputs to the VTA while measuring DA release in the NAc. CIS-FSCV cannot stimulate the afferents as specifically as optogenetic stimulation, but it has an advantage in that it can address questions about presynaptic and postsynaptic receptors within the VTA. While both fluorescent and FSCV approaches have sufficient temporal resolution (subsecond) and sensitivity to DA (1-10 nM) to comparably measure changes in phasic DA release30,31, one advantage FSCV may have over fluorescent monitoring of phasic DA in vivo is that no genetic manipulations are required for recording. Indeed, a CIS-FSCV experiment can be completed within hours, whereas combined optogenetic and fluorescence approaches require sufficient time (weeks) for sufficient expression using viral constructs.
A key benefit of CIS-FSCV is that specific VTA receptor regulation of phasic DA release can be studied in the intact brain, building on other in vivo studies that either measure the electrophysiological properties of VTA neurons or in vitro studies that evaluate the presynaptic regulation of phasic DA release3,12. One caveat of CIS-FSCV is that these recordings must be done in a relatively DA-rich area. This is for two reasons: First, there are some limits to FSCV sensitivity, which can only detect DA concentrations in the nanomolar range and above6,19. Second, FSCV has trouble dissociating norepinephrine from DA, because their cyclic voltammograms are nearly identical. Thus, these studies may be limited to assessing areas with high DA, such as some parts of the medial prefrontal cortex, NAc, striatum, and the olfactory tubercle32. Future studies might be able to employ some of the advances FSCV approaches that allow for better discrimination between DA and NE, as well as other electroactive neurotransmitters such as adenosine33 and serotonin12.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Work was supported by Elizabethtown College (R.J.W, M.L., and L.M.), by a NSF Graduate Fellowship (R.J.W.) and by the Yale School of Medicine (N.A.).
Materials
| Electrode Filling Solution/Supplies | |||
| Micropipette | World Precision Instruments | MF286-5 (28 gauge) | |
| Potassium Acetate | Sigma | 236497-100G | |
| Potassium Chloride | Sigma | P3911-25G | |
| Electrode Supplies | |||
| Carbon fiber | Thornel | T650 | |
| Electrode puller | Narishige International | PE-22 | Note: horizontal pullers can be used as well |
| Glass capillary | A-M systems | 626000 | |
| Insulated wires for electrodes | Weico Wire and Cable Incorporated | UL 1423 | Length; 10 cm; diameter,0.4mm; must get custom made; insulated material should cover 5 cm of the wire |
| Light Microscope (for viewing and cutting electrode) | Fischer Scientific | M3700 | |
| Pin | Phoenix Enterprises | HWS1646 | To be soldered onto the insuled electrode wire and reference electrode; connects to headstage |
| Putty | Alcolin | 23922-1003 | Used to place electrode on while cutting the carbon fiber |
| Scalpal Blade | World Precision Instruments | 500239 | For cutting carbon fiber to the apprpriate length |
| Silver Wire | Sigma | 327026-4G | |
| FSCV Hardware/Software | |||
| Faraday Cage | U-Line | H-3618 (36" x 24" x 42") | |
| Potentiostat | Univ. of N. Carolina, Electronics Facility | ||
| Stimulating electrode | PlasticsOne | MS303/2-A/SPC | when ordering, request a 22 mm cut below pedestal |
| TarHeel HDCV Software | University of North Carolina-Chapel Hill | – | https://chem.unc.edu/critcl-main/criticl-electronics/criticl-electronics-hardware/ for ordering information |
| UEI breakout box | Univ. of N. Carolina, Electronics Facility | https://chem.unc.edu/critcl-main/criticl-electronics/criticl-electronics-hardware/ for ordering information | |
| UEI power supply | Univ. of N. Carolina, Electronics Facility | https://chem.unc.edu/critcl-main/criticl-electronics/criticl-electronics-hardware/ for ordering information | |
| Stimulator Hardware | |||
| Neurolog stimulus isolator | Digitimer Ltd. | DS4 | Neurolog 800A |
| Infusion/Stimulation Supplies | |||
| Infusion Pump | New Era Syringe Pump | NE-300 | |
| Internal Cannula | PlasticsOne | C315I/SPC INTERNAL 33GA | |
| Microliter Syringe | Hamilton | 80308 | |
| Tubing | PlasticsOne | C313CT/ PKG TUBING 023 X 050 PE50 | |
| Surgical Supplies | |||
| Cannula Holder | Kopf Instruments | 1776 P-1 | |
| Cotton Tip Applicators | Vitality Medical | 806 | |
| Electrode Holder | Kopf Instruments | 1770 | |
| Heating Pad | Kent Scientific | RT-0501 | |
| Povidone Iodine | Vitality Medical | 29906-004 | |
| Screws | Stoelting | Bone Anchor Screws/Pkg.of 100 | 1.59 mm O.D., 3.2 mm long |
| Silver wire reference with AgCl | InVivo Metric | E255A | |
| Square Gauze | Vitality Medical | 441408 | |
| Stereotax | Kopf Instruments | Model 902 (Dual Arm Bar) | |
| Histological Supplies | |||
| Formulin | Sigma | 1004960700 | |
| Power supply | BK Precision | 9110 | |
| Sucrose | Sigma | 80497 | |
| Tungsten microelectrode | MicroProbes | WE30030.5A3 | |
| Drugs for infusions | |||
| ((2R)-amino-5-phosphonovaleric acid | Sigma Aldrich | A5282 | |
| N-methyl-D-aspartate | Sigma Aldrich | M3262 | |
| Mecamylamine hydrochloride (M9020-5mg) | Sigma Aldrich | M9020 | |
| Scopolamine hydrobromide (S0929-1g) | Sigma Aldrich | S0929 |
References
- Grace, A. A., Bunney, B. S. The control of firing pattern in nigral dopamine neurons: burst firing. Journal of Neuroscience. 4 (11), 2877-2890 (1984).
- Lester, D. B., et al. Midbrain acetylcholine and glutamate receptors modulate accumbal dopamine release. Neuroreport. 19 (9), 991-995 (2008).
- Lodge, D. J., Grace, A. A. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proceedings of the National Academy of Sciences of the United States of America. 103 (13), 5167-5172 (2006).
- Li, C., et al. Mu Opioid Receptor Modulation of Dopamine Neurons in the Periaqueductal Gray/Dorsal Raphe: A Role in Regulation of Pain. Neuropsychopharmacology. 41 (8), 2122-2132 (2016).
- Zhang, H. Y., et al. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addiction Biology. 22 (3), 752-765 (2017).
- Wickham, R. J., et al. Advances in studying phasic dopamine signaling in brain reward mechanisms. Frontiers in Bioscience. 5, 982-999 (2013).
- Wightman, R. M., et al. Monitoring of transmitter metabolites by voltammetry in cerebrospinal fluid following neural pathway stimulation. Nature. 262 (5564), 145-146 (1976).
- Grace, A. A., Bunney, B. S. The control of firing pattern in nigral dopamine neurons: single spike firing. Journal of Neuroscience. 4 (11), 2866-2876 (1984).
- Mameli-Engvall, M., et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 50 (6), 911-921 (2006).
- Wickham, R., et al. Ventral tegmental area alpha6beta2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology. 229 (1), 73-82 (2013).
- Solecki, W., et al. Differential role of ventral tegmental area acetylcholine and N-methyl-D-aspartate receptors in cocaine-seeking. Neuropharmacology. 75, 9-18 (2013).
- John, C. E., Jones, S. R., Michael, A. C., Borland, L. M. Fast Scan Cyclic Voltammetry of Dopamine and Serotonin in Mouse Brain Slices. Electrochemical Methods for Neuroscience. , (2007).
- Rice, M. E., Cragg, S. J. Nicotine amplifies reward-related dopamine signals in striatum. Nature Neuroscience. 7 (6), 583-584 (2004).
- Espana, R. A., et al. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 214 (2), 415-426 (2011).
- Addy, N. A., et al. The L-type calcium channel blocker, isradipine, attenuates cue-induced cocaine-seeking by enhancing dopaminergic activity in the ventral tegmental area to nucleus accumbens pathway. Neuropsychopharmacology. 43 (12), 2361-2372 (2018).
- Hermans, A., Wightman, R. M. Conical tungsten tips as substrates for the preparation of ultramicroelectrodes. Langmuir. 22 (25), 10348-10353 (2006).
- Borland, L. M., Michael, A. C., Borland, L. M., Michael, A. C. An Introduction to Electrochemical Methods in Neuroscience. Electrochemical Methods for Neuroscience. , (2007).
- Mundroff, M. L., Wightman, R. M. Amperometry and cyclic voltammetry with carbon fiber microelectrodes at single cells. Current Protocols in Neuroscience. 6 (6), 14 (2002).
- Rodeberg, N. T., et al. Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in vivo Fast-Scan Cyclic Voltammetry. ACS Chemical Neuroscience. 8 (2), 221-234 (2017).
- Sabeti, J., Gerhardt, G. A., Zahniser, N. R. Chloral hydrate and ethanol, but not urethane, alter the clearance of exogenous dopamine recorded by chronoamperometry in striatum of unrestrained rats. Neuroscience Letters. 343 (1), 9-12 (2003).
- Masuzawa, M., et al. Pentobarbital inhibits ketamine-induced dopamine release in the rat nucleus accumbens: a microdialysis study. Anesthesia & Analgesia. 96 (1), 148-152 (2003).
- Montague, P. R., et al. Dynamic gain control of dopamine delivery in freely moving animals. Journal of Neuroscience. 24 (7), 1754-1759 (2004).
- Keithley, R. B., et al. Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Analytical Chemistry. 83 (9), 3563-3571 (2011).
- Jackson, B. P., Dietz, S. M., Wightman, R. M. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Analytical Chemistry. 67 (6), 1115-1120 (1995).
- Park, J., Takmakov, P., Wightman, R. M. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. Journal of Neurochemistry. 119 (5), 932-944 (2011).
- Wenzel, J. M., et al. Phasic Dopamine Signals in the Nucleus Accumbens that Cause Active Avoidance Require Endocannabinoid Mobilization in the Midbrain. Current Biology. 28 (9), 1392-1404 (2018).
- Spanos, M., et al. NMDA Receptor-Dependent Cholinergic Modulation of Mesolimbic Dopamine Cell Bodies: Neurochemical and Behavioral Studies. ACS Chemical Neuroscience. 10 (3), 1497-1505 (2019).
- Cheer, J. F., et al. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. Journal of Neuroscience. 24 (18), 4393-4400 (2004).
- Melchior, J. R., et al. Optogenetic versus electrical stimulation of dopamine terminals in the nucleus accumbens reveals local modulation of presynaptic release. Journal of Neurochemistry. 134 (5), 833-844 (2015).
- Sun, F., et al. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell. 174 (2), 481-496 (2018).
- Robinson, D. L., et al. Monitoring rapid chemical communication in the brain. Chemical Reviews. 108 (7), 2554-2584 (2008).
- Park, J., et al. Heterogeneous extracellular dopamine regulation in the subregions of the olfactory tubercle. Journal of Neurochemistry. 142 (3), 365-377 (2017).
- Ganesana, M., Venton, B. J. Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PLoS One. 13 (5), e0196932 (2018).

