Histological Analyses of Acute Alcoholic Liver Injury in Zebrafish
Summary
This protocol describes histological analyses of the livers from zebrafish larvae that have been treated with 2% ethanol for 24 h. Such acute ethanol treatment results in hepatic steatosis and swelling of the hepatic vasculature.
Abstract
Alcoholic Liver Disease (ALD) refers to damage to the liver due to acute or chronic alcohol abuse. It is among the leading causes of alcohol-related morbidity and mortality and affects more than 2 million people in the United States. A better understanding of the cellular and molecular mechanisms underlying alcohol-induced liver injury is crucial for developing effective treatment for ALD. Zebrafish larvae exhibit hepatic steatosis and fibrogenesis after just 24 h of exposure to 2% ethanol, making them useful for the study of acute alcoholic liver injury. This work describes the procedure for acute ethanol treatment in zebrafish larvae and shows that it causes steatosis and swelling of the hepatic blood vessels. A detailed protocol for Hematoxylin and Eosin (H&E) staining that is optimized for the histological analysis of the zebrafish larval liver, is also described. H&E staining has several unique advantages over immunofluorescence, as it marks all liver cells and extracellular components simultaneously and can readily detect hepatic injury, such as steatosis and fibrosis. Given the increasing usage of zebrafish in modeling toxin and virus-induced liver injury, as well as inherited liver diseases, this protocol serves as a reference for the histological analyses performed in all these studies.
Introduction
Alcoholic Liver Disease (ALD), which is caused by alcohol overconsumption, is a major cause of alcohol-related morbidity and mortality. In the United States, nearly half of liver disease deaths involve alcohol1, and ALD is responsible for almost 1 in 3 liver transplants2. ALD has a broad spectrum. Steatosis, which is characterized by excess lipid accumulation in hepatocytes, occurs in the early stage of heavy drinking and is reversible upon cessation of alcohol use. Under the influence of genetic and environmental factors and continuing alcohol intake, hepatic steatosis can progress to alcoholic hepatitis and, eventually, cirrhosis 3. Studies using the rodent ALD models have provided substantial insights into the disease, but they have limitations (reviewed in reference3). Oral feeding of an alcohol diet only causes steatosis in rodents4,5. Development of inflammation and fibrosis requires either a second insult6,7 or chronic intragastric infusion, which is invasive and technically challenging8,9. The teleost zebrafish also develops liver injury in response to both chronic and acute alcohol treatment10,11,12,13,14,15. In particular, the larval zebrafish represents an attractive complementary model organism in which to study acute alcoholic liver injury10,11,13,15. The zebrafish liver is functional and produces key enzymes for ethanol metabolism by 4 days post-fertilization (dpf)13,16,17.Ethanol can be directly added to the water, and exposure to 2% ethanol for 24 h is sufficient to induce hepatic steatosis and fibrogenic responses in zebrafish larvae13,15.
It has been reported that treatment with 2% ethanol for 24 h resulted in a tissue ethanol concentration of 80 mM in zebrafish larvae13. Others have shown that larvae tolerate this concentration and the liver phenotypes seen in the treated animals are specific to ethanol exposure11,13,15,18. However, because 80 mM is nearly lethal in humans19, it is important to evaluate the liver histology of the ethanol-treated zebrafish and determine the physiological relevance to humans.
The rapid external development and translucence of zebrafish larvae make it possible to characterize the action of alcohol within the liver in real-time and in fixed samples. The availability of cell type-specific fluorescent transgenic lines and the recent advances in confocal microscopy facilitate the study of how different liver cell types change their morphology and behavior in response to acute ethanol treatment11,15. However, confocal imaging of the fluorescent transgenic zebrafish cannot completely substitute for Hematoxylin and Eosin (H&E) staining when studying liver histology. Marking all liver cell types at the same time using transgenic zebrafish requires the generation of individual transgenic lines, each labeling one liver cell type with a unique fluorophore. Introducing different transgenic backgrounds into the same fish requires breeding multiple generations, which is time-consuming and costly. Additional immunofluorescence staining is needed to detect extracellular matrix components. H&E staining, on the other hand, simultaneously labels all liver cell types and extracellular matrix components, thus providing an overview of the liver20. Moreover, it readily reveals several histopathological features of liver diseases, such as hepatocyte death, steatosis, and fibrosis. Although H&E is a routine stain in mammalian liver histology, it is not commonly used in zebrafish liver research, and the protocol is less well established.
This work describes a protocol for acute ethanol treatment in zebrafish larvae and for the follow-up histological analyses with H&E staining. The H&E staining protocol can be used in all studies of liver development and function. Moreover, the paraffin sections can be used for immunohistochemistry, as well as for other special stains in liver pathology, including the trichrome stain, reticulin stain, etc.
Protocol
AB WT adult and larval zebrafish were maintained under standard conditions21 in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 86-23, revised 1985); their use was approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital Medical Center (CCHMC).
1. Preparation of Solutions
- Prepare egg water.
- Prepare the stock salt solution by dissolving 40 g of commercial sea salt in 1 L of double-distilled water (ddH2O). Stir until all salts have completely dissolved.
- Prepare 0.1% methylene blue solution by adding 0.1 g of powder to 100 mL of ddH2O.
CAUTION: Methylene blue is an irritant if it comes in contact with the eyes or skin. Always wear a lab coat and gloves when handling the powder. - Combine 28.125 mL of stock salt solution and 7 mL of methylene blue solution in a 50 mL conical tube. Mix well. Add this mixture to 15 L of ddH2O. Shake thoroughly and store at RT.
- Prepare 100 mL of 1 M Tris-HCl by adding 12.1 g of Tris powder to 100 mL of ddH2O. Adjust the pH to 9.0 using hydrochloric acid (HCl).
CAUTION: HCl can cause severe burns and mucous membrane irritation if inhaled. Always wear a lab coat and gloves and handle the stock bottle in a chemical hood. - Prepare 0.4% tricaine (ethyl 3-aminobenzoate methanesulfonate) solution.
- Dissolve 2 g of tricaine powder in 489.5 mL of ddH2O. Add 10.5 mL of the Tris-HCl, pH 9 solution. Mix with a stir bar until all of the powder has dissolved. Adjust to pH 7.0.
CAUTION: Tricaine powder can be a respiratory irritant. Always wear a dust mask when handling the powder. - Aliquot 45 mL of the tricaine solution into 50 mL conical tubes. Keep the solution at 4 °C for immediate use and at -20 °C for long-term storage.
- Dissolve 2 g of tricaine powder in 489.5 mL of ddH2O. Add 10.5 mL of the Tris-HCl, pH 9 solution. Mix with a stir bar until all of the powder has dissolved. Adjust to pH 7.0.
- Prepare Dietrich's fixative by combining 30 mL of 95% ethanol, 10 mL of 37% formaldehyde, 2 mL of glacial acetic acid, and 58 mL of ddH2O in a glass media bottle. Mix well by swirling and store at RT.
CAUTION: Formaldehyde is a suspected carcinogen, and any solution with formaldehyde should be used in a chemical hood. Glacial acetic acid can cause severe skin burns and eye damage. Always wear a lab coat and gloves and handle the stock solution in a chemical hood. Formaldehyde, glacial acetic acid, and 95% ethanol are all flammable liquids and should be stored in a designated flammable cabinet.
NOTE: Dietrich's fixative is stable at RT for 1 year. - Prepare 1 L of 10x Phosphate-buffered Saline (PBS) solution by adding 80 g of sodium chloride (NaCl), 2 g of potassium chloride (KCl), 14.4 g of disodium phosphate (Na2HPO4), and 2.4 g of monopotassium phosphate (KH2PO4) to 800 mL of ddH2O. Adjust to pH 7.4 using sodium hydroxide (NaOH) and add ddH2O to a 1 L final volume. Store this solution at RT after autoclaving on a liquid cycle that purges for 1 min and incubates at 121 °C for 20 min.
- Make 1 L of 1x PBS by diluting 100 mL of the 10x PBS solution in 900 mL of ddH2O. Mix well by shaking and store at RT after autoclaving.
- Prepare 3% agarose in a glass media bottle for embryo mounting.
- Add 3 g of agarose to 100 mL of ddH2O and mix by swirling. Heat the mixture by microwaving to dissolve the agarose, and watch carefully during heating to ensure that there is minimal boiling.
CAUTION: The bottle will likely be hot when removed from the microwave, despite short heating. Use a protective glove or mitt to remove the bottle from the microwave. - Remove the bottle from the microwave. While swirling gently, look for any crystals that are not completely dissolved; if there are no crystals, the solution is ready for use. Store the 3% agarose at RT and heat again before use.
- Add 3 g of agarose to 100 mL of ddH2O and mix by swirling. Heat the mixture by microwaving to dissolve the agarose, and watch carefully during heating to ensure that there is minimal boiling.
- Filter Harris hematoxylin stock solution to remove any solids that have precipitated.
- Fold a coffee filter in half so that the filter creates a cone. Open the side panel so that the liquid runs through the filter, allowing any debris to be caught.
- Place the filter inside a funnel and the funnel into a glass media bottle. Gradually pour the stock hematoxylin through the filter.
CAUTION: Hematoxylin is hazardous in case of eye contact, ingestion, and inhalation. Always wear a lab coat and gloves and handle the stock solution in a chemical hood.
- Prepare 0.05% HCl by adding 125 µL of 12 N HCl to 250 mL of ddH2O.
- Prepare eosin Y-phloxine B solution mixture by combining 25 mL of 1% eosin Y (aq), 2.5 mL of 1% phloxine B (aq), 195 mL of 95% ethanol, and 1 mL of glacial acetic acid in a glass bottle. Mix well by swirling and store at RT
CAUTION: Eosin Y is hazardous in the case of eye contact, ingestion, and inhalation. Always wear a lab coat and gloves and handle the stock solution in a chemical hood. - Prepare 2% ethanol by adding 2 mL of pure ethyl alcohol to 98 mL of egg water.
CAUTION: Ethyl alcohol is an eye irritant. Always wear proper protective equipment when handling ethyl alcohol. It is also flammable and should be handled and stored accordingly.
NOTE: Prepare fresh 2% ethanol each time before conducting the acute ethanol treatment.
2. Perform Acute Ethanol Treatment in Zebrafish Larvae
- To generate embryos, set up crosses with one wildtype male and one WT female fish per mating tank. Separate them by inserting a plastic divider in the mating tank.
NOTE: Set up crosses after the final feeding of the day so that the fish are well fed.- At 8 AM the next morning, pull the divider to allow the pair to mate.
- After 1 h, return the adult fish to their original tank. Collect the embryos by pouring the water containing the embryos into a strainer. Turn the strainer over in a 100 mm Petri dish and wash the embryos into the dish using a wash bottle containing egg water. Place the dish with the embryos in an incubator at 28 °C.
- When embryos reach at least the 4-cell stage (1 hpf), remove any unfertilized embryos and debris in the water with a Pasteur pipette and place the dish with the fertilized embryos in an incubator at 28 °C. Maintain the embryos in the incubator at 28 °C until 96 h post-fertilization.
- Anesthetize the larval fish by adding the tricaine solution to egg water at a 1-10 (volume/volume) ratio.
- Select up to forty 96 hpf larvae that have an inflated swim bladder using a Pasteur pipette and split them evenly into two new petri dishes.
- Take out as much residual egg water as possible. At a density of one larva per mL, add egg water containing 2% ethanol to one dish. Add the same amount of egg water to the other dish to serve as the control.
- Keep the control and ethanol-treated larvae in the fish room for 24 h.
NOTE: Conducting ethanol treatment in a fish room that has a 14 h light/10 h dark cycle results in more consistent and robust liver injury than conducting the experiment in the dark in an incubator. - Collect the larvae in a 1.5 mL centrifuge tube with no more than 20 larvae per tube.
- Remove as much liquid as possible from the larvae and add at least 1 mL (10x tissue volume) of Dietrich's fixative in the chemical hood. Allow the fish to fix on a nutator at room temperature for at least 24 h.
NOTE: Fish are stable in Dietrich's fixative for several weeks.
3. Preparation of Tissue Cassettes and Processing
- Set out the necessary number of tissue cassettes and two blue biopsy pads per cassette. Place one biopsy pad at the bottom of each cassette. Label each cassette in pencil, clearly identifying the sample.
NOTE: Do not use pen or marker to label the cassettes, because the labeling will be lost in later steps. - Embed the larvae in 3% agarose to ensure the consistent orientation of all samples.
- Remove the fixative from the tubes in a chemical hood and wash the larvae 3 times for 5 min each with 1 mL of 1x PBS. Place the tubes on a nutator at RT during the washes.
- During the washes, heat the prepared 3% agarose in water using a microwave to melt the solid. Heat for 30 s at a time and watch carefully to minimize boiling. Keep the liquid agarose on a hot plate set to 90 °C with gentle stirring.
- Using a transfer pipette, transfer up to 8 larvae to a plastic histology mold and remove as much PBS as possible. Using a transfer pipette with the tip cut off, completely fill the mold with 3% agarose.
- Using an insulin syringe, gather the larvae to the center of the mold and push them to the bottom. For sagittal sections, position the larvae in a line, with the heads towards the top of the mold. To ensure that the livers are oriented in a consistent manner, turn the larvae so that the left side of the body is facing down and flat against the bottom of the mold.
NOTE: Because the liver is located on the left side of the body, such positioning minimizes the number of sections that need to be cut before reaching the liver. Keep the larvae as close to each other as possible. - Set the mold to the side for 4-5 min to allow the agarose to set completely. Remove the agarose block from the mold and use a razor blade to trim the agarose around the larvae. Stand the block on end and cut the thickness in half so that the final block is roughly 2-3 mm thick.
- Transfer the small agarose block to the prepared tissue cassette (step 3.1) and place the second biopsy pad on top of the block. Close the cassette and place the fully assembled cassette into a sealable container with freshly prepared 70% ethanol in ddH2O.
NOTE: If the cassettes are not going to be processed immediately, place them in 70% ethanol in a container at 4 °C to avoid a loss of fixation. Cassettes are stable at 4 °C for up to 3 days.
- Tissue processing
NOTE: Samples can be submitted to a histology or pathology laboratory equipped with a tissue processor for processing the cassettes in paraffin. Request a standard O/N processing program rather than a short program so that the paraffin can fully penetrate the agarose.- Process the tissue cassettes by transferring them through a dilution series of ethanol, with an increasing amount of alcohol, to dehydrate the tissue, as follows: 70% ethanol 2 times, 45 min each; 80% ethanol, 45 min; 95% ethanol, 2 times, 45 min each; 100% ethanol, 2 times, 45 min each.
- Replace the alcohol with a solvent (100% xylene, 2 times, 45 min each) in order for the paraffin to infiltrate the tissue.
CAUTION: Xylene is an irritant and is harmful if inhaled. Be sure to always use a chemical hood. Xylene is flammable and must be stored accordingly. - Incubate the cassettes in paraffin O/N in an incubator at 60-65 °C. Keep the cassettes warm until embedding.
- Embed the processed agarose blocks into paraffin.
- Take out one cassette from the warming drawer of the embedding machine and remove the lid of the cassette and the top biopsy pad from the cassette. Fill a histology mold with liquid paraffin and keep it on the warm plate on the embedding machine.
- Using warm forceps, pick up the agarose block from the cassette and transfer it to the histology mold with paraffin. Make sure that the side of the agarose block with the fish closest to the surface is facing down. Throw away the bottom biopsy pad and transfer the entire histology mold to the cool plate on the embedding machine.
- Using forceps, quickly position the agarose block at the center of the mold; place the block at the bottom of the mold. Once the block is properly placed, put the bottom of the cassette on top of the histology mold and fill it halfway with liquid paraffin. Place the mold directly on the 4 °C plate and do not disturb the blocks for at least 10-15 min to allow the paraffin to solidify. While the first block is setting up, repeat this process for the remaining blocks.
- Once the paraffin is set for all of the blocks, remove the histology mold from the paraffin block by pressing gently on the mold to loosen it. These paraffin blocks can be stored at room temperature indefinitely.
4. Sectioning of Paraffin Blocks
- The day before sectioning, face-in the paraffin block using a microtome to remove excess paraffin covering the tissue. Section 5 µm at a time until the tissue is exposed at the surface of the paraffin block. Stop and discard all the sections that are cut. Soak the blocks face down in 1x PBS at 4 °C O/N.
NOTE: The blocks should be soaked for at least 8 h. Start the soaking at the end of the day. If the blocks are left in 1x PBS for too long, the tissue can swell and become distorted. - Remove one block at a time from the 1x PBS and cut 5-µm sections using a microtome. Separate the ribbon of sections from the blade by gently pulling the last section away from the blade using forceps or a brush. Pick up the ribbon by the last section using the forceps and transfer it to a water bath at 42 °C. Allow the ribbons to float on the surface of the water for at least 5 min.
NOTE: When transferring sections, do not let the forceps touch the water while still in contact with the paraffin. Otherwise, the paraffin will melt onto the forceps and make separation very difficult. If necessary, tease apart the sections into smaller groups using clean forceps so that they can easily fit onto the slides. Gently touch the seam between sections to create separation without damage. - Put a charged slide into the water at a 45-degree angle and carefully position it underneath the group of sections to be collected. Carefully lift the slide from the water and allow the sections to attach to the slide.
- Blot any excess water from the sections using lint-free tissue. Place the slide in a slide holder or box. Continue to section the block until the desired tissue has been collected. Repeat the sectioning process for all blocks.
- Bake the slides in a 55 °C incubator or oven for 3-16 h to melt the paraffin. Remove the slides from the oven and allow them to cool before beginning the staining.
NOTE: Paraffin sections can be stored at RT indefinitely, both before and after baking.
5. Hematoxylin and Eosin Staining of Paraffin Sections
- Deparaffinize the slides by dipping them in 100% xylene for 15 min; change it to fresh 100% xylene for another 15 min.
- Rehydrate the slides by dipping them through the following series of graded ethanol until the liquid runs cleanly off the slides. For each solution, dip 8-10 times, 2 s per dip: 100% ethanol, 100% ethanol, 95% ethanol, 95% ethanol, 70% ethanol, 50% ethanol, 30% ethanol, and deionized water.
NOTE: The protocol can be stopped here, and the slides can be left at RT in water for several hours. If necessary, the slides can be stored at 4 °C in water O/N. - Place the slides in 100% filtered Harris hematoxylin for 4 min. Immediately transfer them back to the container with deionized water. Run deionized water into the back corner of the container farthest away from the sections. Empty the container periodically until the water is no longer purple.
NOTE: Do not let the water run directly onto the slides, as the sections may come off the slides. - Quickly check the hematoxylin intensity on a dissecting microscope using gooseneck lights; do not allow the slides to dry. If the stain has reached the desired intensity, continue to the next step. If the color is not dark enough, place the slides in 100% hematoxylin for 1 min and repeat the water washes before checking again.
NOTE: The hematoxylin needs to be dark enough so that the color is not lost during the eosin staining. However, if hematoxylin staining is seen in the cytoplasm, the sections are overstained. - Dip the slides twice in 0.05% HCl and immediately transfer them back to the container with clean deionized water. Empty the water and refill the container with water twice.
- Transfer the slides to 95% ethanol for 30 s and then transfer them to a new container with 95% ethanol for 30 s.
- Place the slides into the eosin Y-phloxine B solution for 2 min. Transfer the slides back to the previous 95% ethanol container and quickly check the intensity of the color under the dissecting microscope. If the staining is sufficient, proceed to the next step. If not, return to the eosin solution for 30 s, check again, and repeat as necessary.
NOTE: Sufficient eosin staining is bright pink and appears in distinct contrast to the hematoxylin stain. Be sure to wipe any solution off the back of the slides when checking under the microscope so that no false color is observed. Because eosin is made up of 95% ethanol, prolonged periods of time in 95% ethanol while checking color intensity may leach out some of the color. - Transfer the slides to 100% isopropanol for 15 s. Replace with fresh 100% isopropanol and place the slides back in the isopropanol for another 15 s. Repeat this process for a total of 6 isopropanol washes.
CAUTION: Isopropanol is an eye and respiratory irritant. Always be sure to wear proper protective equipment and avoid splashing. It is also highly flammable and should be stored and handled accordingly.
NOTE: To save on reagents, discard only the first (pinkest) isopropanol wash. Keep the other five wash solutions in bottles numbered 1 through 5, to be reused for the next staining. When reusing washes, start with the bottle numbered "1" and discard after use. Once wash "2" has been used, pour it into bottle "1," and so on. The final wash should always be fresh isopropanol. - Place the slides in 100% xylenes for 3 min. Remove one slide at a time and place a coverslip.
- Add sufficient mounting medium to cover the sections and dip them in 100% xylene.
NOTE: This step will smooth the surface of the mounting medium and remove any bubbles. - Apply a coverslip to the bottom of the slide.
NOTE: The mounting medium should pull the slide into position. If the coverslip is not straight or completely seated, gently tap it into place. - Blot any excess mounting medium on a paper towel until only a thin line is seen. Dip a tissue wipe into the xylene and wipe the back of the slide to remove any medium that has dripped. Place the slide flat on a sturdy but mobile surface, like a piece of cardboard. Coverslip all the remaining slides in the same fashion. Allow the xylene to evaporate in the hood for 10 min.
NOTE: Any materials contaminated with xylene should be removed and discarded in a sealed container in the hood to prevent any vapors from escaping.
- Add sufficient mounting medium to cover the sections and dip them in 100% xylene.
- Allow the mounting medium to harden at RT O/N.
6. Imaging and Storage of Stained Slides
- Image sections on a compound inverted microscope18.
NOTE: Slides can be kept at room temperature indefinitely.
Representative Results
10% buffered formalin and 4% paraformaldehyde (PFA) are two of the most common fixatives used for histology practices. However, they do not give optimal fixation results for zebrafish liver tissue (Figure 1 and Table 1). Fixation with 10% formalin or 4% PFA often results in shrinkages, creating large gaps between the liver and surrounding tissues (Figure 1A, B; Figure 1B provides an example of tissue shrinkage). Portions of the liver tissue may also fall out of the section. The cytoplasm of the hepatocytes is often lost (Figure 1A, 1B). Both fixatives can cause distortion of the hepatocytes, as they either shrink or swell and no longer maintain the columnar morphology that is characteristic for epithelial cells. One additional issue with the PFA fixation is that there are spaces between the hepatocytes that are not real, which cause the tissue to look fractured (Figure 1B). Furthermore, when either of these two fixatives is used, the hepatocytes are stained well with hematoxylin, but less so with eosin (Figure 1A). The acid-based Dietrich's fixative overcomes the issues with the formalin and PFA fixatives (Figure 1C).
In human ALD, steatosis, which is composed of small- and large-droplet fat, is most prominent near the central vein and extends outward toward the portal triad with increasing severity22. In zebrafish, treatment with 2% ethanol from 96-120 hpf also induces hepatic steatosis, which is implicated by the excessive deposition of round droplets in the hepatocytes (Figure 2B, arrows). However, the droplets do not exhibit a similar distribution pattern as seen in human ALD, because there is no clear distinction of portal and central veins in the zebrafish liver23. The hepatic blood vessels in the ethanol-treated larval liver are swollen compared to those in the control liver (Figure 2B, asterisks).
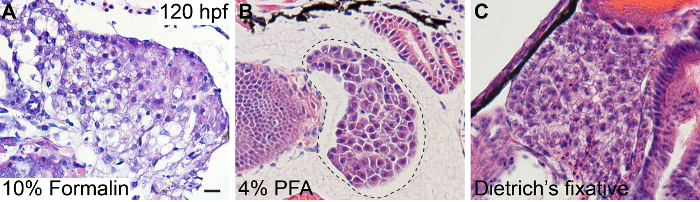
Figure 1: Comparisons of H&E Staining in the Livers of the Zebrafish Larvae that were Fixed with Differert Fixatives at 120 hpf. (A) 10% Formalin at RT O/N; (B) 4% PFA at 4 °C O/N; (C) Dietrich's fixative at RT for 24 h. With 10% formalin, the hepatocytes often lose their cytoplasm (A). The tissue is not stained properly with eosin and appears purple. After fixation with 4% PFA, gaps are seen between the hepatocytes (B). 4% PFA causes the liver tissue to shrink, so there appears to be a large gap between the liver and the surrounding tissues. The dashed line in B marks the border of the surrounding tissues. Dietrich's fixative provides the optimal result among the three (C). Scale bar = 20 µm. Please click here to view a larger version of this figure.
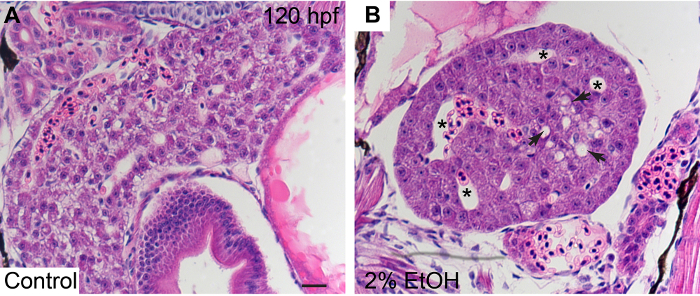
Figure 2: Acute Ethanol Treatment Causes Hepatic Steatosis and Blood Vessel Swelling in Zebrafish Larval Livers. (A) H&E staining of the liver in a control, untreated, WT larva. (B) H&E staining of the liver in a wil type larva that was treated with 2% ethanol from 96 – 120 hpf. Both animals were fixed with Dietrich's fixative at 120 hpf. Arrows in (B) point to the hepatocytes with excessive deposition of round droplets. Asterisks mark the swollen hepatic blood vessels. Scale bar = 20 µm. Please click here to view a larger version of this figure.
| 10% formalin | 4% PFA | Dietrich’s fixative | |
| Fixation condition | At least 24 h at RT | 3 h at RT or O/N at 4 °C | At least 24 h at RT |
| Sample storage post fixation | Indefinitely at RT | Up to 2 weeks at 4 °C | Up to 2 months at RT |
| Compatibility with genomic DNA extraction | Yes | Yes | No |
| Shrinkages | Yes | Yes | Minimum |
| Distortion of cells | Yes | Yes | Minimum |
| Loss of cellular details | Yes | Modest | Minimum |
| Balanced H&E stain | Weak eosin stain | Weak eosin stain | Yes |
| Compatibility with immunohistochemistry | Yes | Yes | Yes |
Table 1: Comparisons of 10% formalin, 4% PFA, and Dietrich's Fixatives for Zebrafish Liver Histology.
Discussion
The current protocol describes a detailed procedure for acute ethanol treatment in zebrafish larvae and the subsequent histopathological analyses with H&E staining. Acute ethanol treatment should be conducted at no earlier than 96 h post-fertilization, as this is the stage at which the zebrafish liver starts to express alcohol-metabolizing enzymes13. 2% ethanol is the maximal dose that larvae can tolerate13,14. The ethanol-treated larvae begin to show hepatic steatosis by 8 h of treatment, and the percentages of larvae developing steatosis continue to rise until 24 h of continuous treatment14. Zebrafish larvae absorb nutrients exclusively from the yolk until 120 hpf. Therefore, ethanol treatment beyond 120 hpf is not recommended, because fasting can also contribute to steatosis14. Compared to protocols published by other laboratories13,24, the main modification made in the current protocol is that the ethanol treatment is conducted in a fish facility that has a 14 h light/10 h dark cycle. This results in more consistent and robust steatotic responses than those seen when the treatment is conducted in the dark in an incubator. This may be related to the fact that the lipid metabolic genes in the zebrafish liver show daily rhythm expression in response to the light/dark cycle25.
ALD is a chronic disease and takes years of alcohol abuse. The critical limitation of the current ethanol treatment protocol is that it only triggers the acute effects of alcohol on the liver. Treatment with such a high ethanol concentration for more than 48 h causes high mortality, preventing the study of chronic liver injury. A recent study found that continuous treatment of adult zebrafish with 1% ethanol for up to three months led to steatosis, steatohepatitis, and fibrosis12. One promising future direction could be to identify a potential modulator of ALD using the acute ethanol treatment protocol and then to validate its effect in the chronic injury model.
H&E staining is performed to evaluate the hepatocellular damages that are induced by acute ethanol treatment. Due to the ease of performing fluorescence imaging in zebrafish, H&E staining is not routinely used in zebrafish liver histology, and the procedure is less well described. The current protocol provides a step-by-step description of H&E staining in the larval liver. Choosing the correct fixative is the first and most essential step for H&E staining. Although 10% buffered formalin and 4% PFA are commonly used in histology, they both cause tissue shrinkages and portions of the liver falling out of the section. 10% formalin fixation leads to the loss of cytoplasm in the hepatocytes. 4% PFA fixation results in artificial gaps between the hepatocytes. Eosin staining seems to be much weaker than hematoxylin staining in the livers that are fixed with either fixative. The acid-based Dietrich's fixative is more suited for H&E staining of the zebrafish liver, as it preserves cellular details and minimizes shrinkage. It also seems to penetrate fatty tissue, such as the liver, faster than formalin and PFA. The staining with hematoxylin and eosin is more balanced. One caveat of Dietrich's fixative is that it is not compatible for genomic DNA extraction. In a trial experiment, genomic DNA was extracted from the larvae that were fixed using 10% formalin, 4% PFA, or Dietrich's fixative26. The larvae were incubated in 50 µL of 50 mM NaOH at 95 °C for 20 min and were then cooled to 4 °C. 5 µL of 1 M Tris-HCl, pH 8.0 were then added to neutralize the basic solution. After a brief centrifugation, the supernatant was used in PCR. With the same PCR primers and PCR program, the genomic DNA from both the formalin- and PFA-fixed larvae yielded PCR products with the predicted sizes, whereas the genomic DNA from the Dietrich's fixative-fixed larvae failed to yield any PCR products.
H&E staining can be conducted on both paraffin sections and frozen sections. However, paraffin sections have the following advantages over frozen sections: 1) Whereas paraffin sections can be stored at room temperature indefinitely, frozen sections can only be stored at -80 °C for up to a year. 2) For frozen sections, the formation of ice crystals within the cells may perturb the cell morphology and subcellular detail. Moreover, frozen sections are often thicker than paraffin sections. This can result in poor images of tissue morphology compared to those produced from paraffin sections.
When preparing paraffin blocks for liver tissues, the current protocol embeds the larvae in agarose. The larvae are positioned laterally, with the left side of the body facing down and lying flat against the bottom of the mold. This ensures the consistent orientation of the livers, so that when sections are cut sequentially, equivalent regions of the liver can be compared from fish to fish27. Another key step that ensures successful H&E staining is the color development. It is crucial to check the staining frequently until the desired color intensity is reached.
H&E staining should be used to obtain a preliminary assessment of liver injury. The ethanol-treated larvae show excessive deposition of round droplets in the hepatocytes, suggestive of steatosis13,14,18. Labeling with lipid dyes, such as Oil Red O and Nile Red, is necessary to confirm that these droplets are indeed lipids. The hepatic blood vessels in the treated animals appear dilated and swollen. Scanning electron microscopy and transmission electron microscopy should be performed to examine the ultrastructural changes in the sinusoids. It has been previously reported that extracellular matrix protein deposition is increased in the ethanol-treated zebrafish liver, as detected by immunofluorescence18. However, given that the expression levels of fibrogenic genes are only modestly increased in the treated fish18, H&E may not be sensitive enough to detect such a small amount of extracellular matrix proteins. The same fixative and staining methods were tested on the chronically injured adult zebrafish livers and were sufficient for detecting fibrosis (Yin, unpublished data).
Although the current protocol is tailored to the examination of liver histology in zebrafish larvae, it has a broader application to the zebrafish research community, as the same protocol can be applied to other tissues and to adult zebrafish.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Dr. Katy Murray at the Zebrafish International Resource Center; Dr. Stacey Huppert and Kari Huppert at CCHMC, for their helpful advice on the protocol; and CCHMC veterinary service, for the fish care. This work was supported by NIH grant R00AA020514 and a research grant from the Center for Pediatric Genomics at CCHMC (to C.Y.). It was also support in part by NIH grant P30 DK078392 (Integrative Morphology Core) of the Digestive Disease Research Core Center in Cincinnati.
Materials
| 1.5 mL centrifuge tubes | E & K Scientific | 280150 | |
| 15 mL conical tubes | VWR International | 89039-664 | |
| 50 mL conical tubes | VWR International | 89039-658 | |
| 95% ethanol | Decon Labs, Inc. | 2801 | Flammable |
| Acetic acid | Newcomer Supply | 10010A | Irritant |
| Agarose | Research Products International | 9012-36-6 | |
| Aluminum jar rack holder | Newcomer Supply | 5300JRK | |
| Bacteriological petri dishes with lid | Corning | 351029 | |
| Biopsy pads | Simport | M476.1 | |
| Charged slides | Fisher Scientific | 12-550-16 | |
| Clear mounting media | Fisher Scientific | 8310-16 | Can be substituted with other clear mounting media |
| Commercial sea salts | Instant Ocean | SS15-10 | |
| Disposable microtome blades | Fisher Scientific | 4280L | |
| Dissecting microscope | Leica Biosystems | Leica Mz 95 | |
| Enclosed tissue processor | Leica Biosystems | ASP300 S | |
| Eosin-Phloxine stain set | Newcomer Supply | 1082A | |
| Ethyl alcohol | Sigma-Aldrich | E7023 | Flammable |
| Formaldehyde solution, ACS reagent, 37 WT. % in H20, contains 10-15% methanol as stabilizer (to prevent polymerization) | Sigma-Aldrich | 252549 | A suspected carcinogen; irritant |
| Formalin, Buffered, 10% | Fisher Scientific | SF100-4 | A suspected carcinogen; irritant |
| Graduated media bottle | VWR International | 16159-520 | |
| Harris hematoxylin | Poly Scientific R&D Corp. | s212 | Irritant |
| Histology molds | Sakura Finetek USA Inc | 4557 | |
| Hot plate/Stirrer | VWR International | 47751-148 | |
| Hydrochloric acid | Fisher Scientific | A144 | Irritant |
| Incubator | VWR International | 97058-220 | |
| Insulin syringes | BD Medical | BD-309301 | |
| Inverted compound microscope | Carl Zeiss Microscopy | 491912-9850-000 | |
| Isopropanol | Newcomer Supply | 12094E | Flammable |
| Methylene blue | Sigma-Aldrich | M9140 | Irritant |
| Microtome | Leica Biosystems | Leica Jung BioCut 2035 | |
| Nutating mixer | VWR International | 82007-202 | |
| Paraformaldehyde | Sigma-Aldrich | P6148-1KG | A suspected carcinogen; irritant |
| Pasteur pipet | VWR International | 53283-916 | |
| Pipette pump (10 mL) | VWR International | 53502-233 | |
| Potassium chloride (KCl) | Sigma-Aldrich | P9541 | |
| Potassium phosphate, monobasic (KH2PO4) | Sigma-Aldrich | P9791 | |
| Razor blades | Grainger | 4A807 | |
| Slide Staining Kit | Newcomer Supply | 5300KIT | |
| Sodium chloride (NaCl) | Sigma-Aldrich | S3014 | |
| Sodium hydroxide (NaOH) | Fisher BioReagents | S318-500 | Very hazardous |
| Sodium phosphate, dibasic (Na2HPO4) | Sigma-Aldrich | S3264 | |
| Stainless steel strainer (5 inch diameter) | Adaptive Science Tools | L0906045in | |
| Tissue cassettes | Simport | M505.12 | |
| Tissue embedding center | Sakura Finetek USA Inc | #5100 | |
| Tissue wipers, 1-Ply | Fisher Scientific | 06666A | |
| Transfer pipets | Fisher Scientific | 137117M | |
| Tricaine powder/Ethyl 3-aminobenzoate methanesulfonate salt | Sigma-Aldrich | A5040 | Irritant |
| Tris base, primary standard and buffer | Sigma-Aldrich | T1503 | |
| Wash bottle, low-density polyethylene, wide mouth | Nalge Nunc International | 2402-0750 | |
| Xylenes | Fisher Scientific | X3S-4 | Irritant |
References
- Yoon, Y. H., Chen, C. M., Yi, H. Y. . Surveillance report #100: Liver cirrhosis mortality in the United States: National, State, and regional trends. , 2000-2011 (2014).
- Singal, A. K., et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 95 (5), 755-760 (2013).
- Louvet, A., Mathurin, P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 12 (4), 231-242 (2015).
- Ki, S. H., et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 52 (4), 1291-1300 (2010).
- Tsuchiya, M., et al. Interstrain differences in liver injury and one-carbon metabolism in alcohol-fed mice. Hepatology. 56 (1), 130-139 (2012).
- Koteish, A., Yang, S., Lin, H., Huang, X., Diehl, A. M. Chronic ethanol exposure potentiates lipopolysaccharide liver injury despite inhibiting Jun N-terminal kinase and caspase 3 activation. J Biol Chem. 277 (15), 13037-13044 (2002).
- Leo, M. A., Lieber, C. S. Hepatic fibrosis after long-term administration of ethanol and moderate vitamin A supplementation in the rat. Hepatology. 3 (1), 1-11 (1983).
- Tsukamoto, H., et al. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 5 (2), 224-232 (1985).
- Tsukamoto, H., Mkrtchyan, H., Dynnyk, A. Intragastric ethanol infusion model in rodents. Methods Mol Biol. 447, 33-48 (2008).
- Howarth, D. L., Passeri, M., Sadler, K. C. Drinks like a fish: using zebrafish to understand alcoholic liver disease. Alcohol Clin Exp Res. 35 (5), 826-829 (2011).
- Howarth, D. L., Yin, C., Yeh, K., Sadler, K. C. Defining hepatic dysfunction parameters in two models of fatty liver disease in zebrafish larvae. Zebrafish. 10 (2), 199-210 (2013).
- Lin, J. N., et al. Development of an Animal Model for Alcoholic Liver Disease in Zebrafish. Zebrafish. , (2015).
- Passeri, M. J., Cinaroglu, A., Gao, C., Sadler, K. C. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology. 49 (2), 443-452 (2009).
- Tsedensodnom, O., Vacaru, A. M., Howarth, D. L., Yin, C., Sadler, K. C. Ethanol metabolism and oxidative stress are required for unfolded protein response activation and steatosis in zebrafish with alcoholic liver disease. Dis Model Mech. 6 (5), 1213-1226 (2013).
- Yin, C., Evason, K. J., Maher, J. J., Stainier, D. Y. The bHLH transcription factor Hand2 marks hepatic stellate cells in zebrafish: Analysis of stellate cell entry into the developing liver. Hepatology. , (2012).
- Lassen, N., et al. Molecular cloning, baculovirus expression, and tissue distribution of the zebrafish aldehyde dehydrogenase 2. Drug Metab Dispos. 33 (5), 649-656 (2005).
- Reimers, M. J., Hahn, M. E., Tanguay, R. L. Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol dehydrogenase genes but have distinct functional characteristics. J Biol Chem. 279 (37), 38303-38312 (2004).
- Zhang, C., Ellis, J. L., Yin, C. Inhibition of vascular endothelial growth factor signaling facilitates liver repair from acute ethanol-induced injury in zebrafish. Dis Model Mech. , (2016).
- Vonghia, L., et al. Acute alcohol intoxication. Eur J Intern Med. 19 (8), 561-567 (2008).
- Wittekind, D. Traditional staining for routine diagnostic pathology including the role of tannic acid. 1. Value and limitations of the hematoxylin-eosin stain. Biotech Histochem. 78 (5), 261-270 (2003).
- Westerfield, M. . The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio). , (2007).
- Theise, N. D. Histopathology of alcoholic liver disease. Clinical Liver Disease. 2 (2), (2013).
- Lorent, K., et al. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 131 (22), 5753-5766 (2004).
- Huang, M., Xu, J., Shin, C. H. Development of an Ethanol-induced Fibrotic Liver Model in Zebrafish to Study Progenitor Cell-mediated Hepatocyte Regeneration. J Vis Exp. (111), (2016).
- Paredes, J. F., Lopez-Olmeda, J. F., Martinez, F. J., Sanchez-Vazquez, F. J. Daily rhythms of lipid metabolic gene expression in zebra fish liver: Response to light/dark and feeding cycles. Chronobiol Int. 32 (10), 1438-1448 (2015).
- Meeker, N. D., Hutchinson, S. A., Ho, L., Trede, N. S. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques. 43 (5), 610-614 (2007).
- van der Velden, Y. U., et al. The serine-threonine kinase LKB1 is essential for survival under energetic stress in zebrafish. Proc Natl Acad Sci U S A. 108 (11), 4358-4363 (2011).

