Cannula Implantation into the Cisterna Magna of Rodents
Summary
Here we describe a protocol to perform cisterna magna cannulation (CMc), a minimally invasive way to deliver tracers, substrates and signaling molecules into the cerebrospinal fluid (CSF). Combined with different imaging modalities, CMc enables glymphatic system and CSF dynamics assessment, as well as brain-wide delivery of various compounds.
Abstract
Cisterna magna cannulation (CMc) is a straightforward procedure that enables direct access to the cerebrospinal fluid (CSF) without operative damage to the skull or the brain parenchyma. In anesthetized rodents, the exposure of the dura mater by blunt dissection of the neck muscles allows the insertion of a cannula into the cisterna magna (CM). The cannula, composed either by a fine beveled needle or borosilicate capillary, is attached via a polyethylene (PE) tube to a syringe. Using a syringe pump, molecules can then be injected at controlled rates directly into the CM, which is continuous with the subarachnoid space. From the subarachnoid space, we can trace CSF fluxes by convective flow into the perivascular space around penetrating arterioles, where solute exchange with the interstitial fluid (ISF) occurs. CMc can be performed for acute injections immediately following the surgery, or for chronic implantation, with later injection in anesthetized or awake, freely moving rodents. Quantitation of tracer distribution in the brain parenchyma can be performed by epifluorescence, 2-photon microscopy, and magnetic resonance imaging (MRI), depending on the physico-chemical properties of the injected molecules. Thus, CMc in conjunction with various imaging techniques offers a powerful tool for assessment of the glymphatic system and CSF dynamics and function. Furthermore, CMc can be utilized as a conduit for fast, brain-wide delivery of signaling molecules and metabolic substrates that could not otherwise cross the blood brain barrier (BBB).
Introduction
Cerebrospinal fluid (CSF) bathes the central nervous system (CNS) throughout the ventricular system and along the subarachnoid spaces, an anatomically defined space in continuum with the ventricles, which surrounds the brain and the spinal cord. One of the main functions of the CSF is to provide a route for clearance of metabolites and solutes from the brain parenchyma. Clearance is facilitated via the recently discovered glymphatic system1, the brain analog to the peripheral lymphatic system. Herein, we describe and discuss the cisterna magna cannulation (CMc), a minimally invasive method for the direct delivery of molecules into the CSF. CMc is the key method for studying the glymphatic function. Furthermore, CMc can also be applied for the study of CSF dynamics and for a fast, brain-wide delivery of non-blood brain barrier (BBB) permeable molecules into the brain parenchyma, along the perivascular space.
The CMc exploits physiological principles of CSF movement dynamics through the CNS to deliver labeled tracer molecules or drugs into the CSF-filled space of the cisterna magna (CM). Molecules are injected through a cannula implanted into the atlanto-occipital dural membrane covering the CM. Molecules are then carried by CSF bulk flow into the brain parenchyma via the paravascular space1. Tracer or contrast agent injected via the CMc follows the movement of CSF, which allows the assessment of CSF movement and glymphatic influx by quantifying intensity levels of labeled molecules that enter the brain parenchyma. CMc is compatible with different imaging techniques including epifluorescence, 2-photon microscopy, and magnetic resonance imaging (MRI). Also, this assessment can be performed both in vivo or ex vivo. Importantly, CMc allows for the visualization of the glymphatic system under anesthesia or during natural sleep, as well as in awake, freely moving animals.
The CMc technique can be utilized to study different aspects of fluid dynamics in the CSF, but has proven to be particularly useful for studying the glymphatic system. Glymphatic activity drives the convective flow of CSF from the periarterial space via aquaporin-4 (AQP-4) water channels, which are tethered in the membrane of astrocytic vascular-wrapping endfeet. The convective flow enables the interchange of CSF and interstitial fluid (ISF) within the brain parenchyma. CSF/ISF containing metabolic waste and solutes is then removed from the brain parenchyma via the perivenous space2,3. Ultimately, CSF/ISF reaches the periphery via the recently described dural lymphatic vessels4,5. The glymphatic system has been shown crucial for the clearance of harmful waste metabolites such as amyloid-β2. Further, glymphatic clearance is impaired in aging6, after traumatic brain injury7, and in animal models of diabetes8 and Alzheimer's disease9. Notably, glymphatic activity is state dependent, showing significantly higher activity during sleep or anesthesia in comparison to wakefulness1. Indeed, young anesthetized animals exhibit the highest glymphatic activity. Thus, experimental quantification of glymphatic activity is critical when studying its role in health and disease.
Several studies have addressed CSF dynamics and its interchange with interstitial fluid (ISF) in the brain parenchyma. However, the methods by which labeled molecules are delivered are rather invasive, triggering brain parenchyma damage and changes in the intracranial pressure (ICP) (see review10). Some examples are intraventricular or intraparenchymal injections which involve craniotomy or drilling of a burr hole in the skull. These procedures have been shown to alter ICP, thus disrupting glymphatic function2. Also, such invasive methods induce astrogliosis and increase AQP-4 immunoreactivity in the brain parenchyma damaged area and its surroundings11,12. As astrocytes and AQP-4 are key elements of the glymphatic system, the CMc is the method of choice for its studies. The major advantages of CMc in comparison to more invasive procedures are the maintenance of an intact skull and brain parenchyma, avoiding ICP alterations and astrogliosis, respectively. Thus, CMc in conjunction with different imaging tools opens for a wide range of possibilities to study not only the glymphatic system, but also the dynamics and mechanisms of fluid flow in homeostasis, as well as in animal models of neurological diseases.
The cisterna magna cannulation (CMc) procedure allows easy and direct access to the cerebrospinal fluid (CSF). By injecting different molecules (e.g. fluorescent tracers, MRI contrast agents) the experimenter can track their movement within the CSF compartment and assess the activity of the glymphatic system. The following protocol describes both the acute CMc, for injections immediately following the surgery, and chronic implantation of the cannula, in which the animal recovers from the surgical procedure for a later injection. The most important difference between the acute and chronic implantation is that the chronic implantation allows for the study of glymphatic activity in awake mice.
Protocol
All procedures were performed in accordance with the European Directive 2010/63/EU for animal research and were approved by the Animal Experiments Council under the Danish Ministry of Environment and Food (2015-15-0201-00535).
1. Procedure for Cannulation
- Cannula preparation
NOTE: Avoid touching the cannula with non-sterile gloves.- Break off the beveled metal tip of a 30G dental needle using a needle holder.
- Using a needle holder, prepare the cannula by inserting the beveled metal tip (approximately 0.3 cm) into a 30-cm length of PE10 tubing (Polyethylene Tubing 0.024" OD x 0.011" ID) filled with aCSF (126 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 2 mM CaCl2, 10 mM glucose, 26 mM NaHCO3; pH 7.4 when gassed with 95% O2 and 5% CO2).
- Flush the cannula with aCSF using a 1 mL syringe fitted with a 30G needle (30 G x ½" 0.3 x 12).
- Surgical procedure
NOTE: The chronic CM cannulation allows the animals to recover from the surgical procedure. CM injections are made the day following cannula implantation and, importantly, can be performed in either anesthetized or awake animals. Since this is a recovery surgery, procedures should be carried out under sterile conditions.- Weigh the mouse (C57BL/6JRj, both sexes, 8 weeks) and anesthetize it with a mixture of ketamine and xylazine (100 mg/kg; 10 mg/kg, respectively) via intraperitoneal (i.p.) injection. If necessary, redose the mouse with a half dose of ketamine (50 mg/kg) during the surgical procedure. Alternatively, for chronic cannulation, anesthetize mouse by placing it into an isoflurane induction chamber at 2.5 – 3% isoflurane, in circa 1 L/min O2. In this case, use a nose cone to sustain isoflurane anesthesia at 1.5 – 2% throughout the surgery.
- When toe pinch reflexes cease, and the respiration becomes slow and steady, place the animal in a stereotaxic frame over a heating pad.
- Apply ophthalmic ointment. Repeat during surgery whenever necessary.
- Shave the head and neck of the mouse, remove fur, and sterilize exposed skin first with an alcohol swab and then two times with chlorhexidine (0.5%) or iodine solution (2%). Repeat the sterilization two more times.
NOTE: Change the surgical drape to remove debris and hair after shaving. Then drape the animal to protect the sterile field. - For the chronic cannulation, administer 0.5 – 1 ml lidocaine/bupivacaine (1 mg/ml and 0.25 mg/ml, respectively) subcutaneously (s.c.) at the incision site. Administer buprenorphine (0.05 mg/kg; s.c.) for post-surgical analgesia.
- Fix the mouse in the stereotaxic frame. After ensuring fixation, either intraural or by the zygomatic arch, tilt the head slightly so that it forms an angle of 120° to the body (Figure 1E).
- Find the part of the skull protruding immediately above the neck muscles – the occipital crest. Lift the overlying skin using a pair of tweezers, and cut an almond shaped piece of skin of approximately 1 cm along the midline. Use cotton swabs or eye spears to control any resultant bleeding.
- Using the occipital crest as a reference point, pull apart the superficial connective tissue to expose the neck muscles below.
- Separate the muscles at the midline by carefully running the forceps down the middle of the incision site in the anterior-to-posterior axis. With a pair of curved forceps in each hand, join the tips in the middle near the bottom of the skull and pull the muscles aside.
NOTE: This should expose the CM, which appears as a tiny inverted triangle, outlined by the cerebellum above and medulla below, behind the translucent dural membrane (Figure 1B and 1C). - Using a surgical eye spear or cotton swab, wipe the dural membrane covering the CM.
- Insertion of the cannula into the CM
- Remove the cannula from the aCSF-filled syringe, keeping the 30G needle attached to the rear end of the tubing.
- Attach the 30G needle to a distilled water-filled 100 µL syringe connected to a syringe pump.
- Insert an air bubble of approximately 1 cm into the cannula by withdrawing air with the aid of a syringe pump.
- Using a syringe pump, withdraw 12 μL of desired CSF tracer into the cannula.
- Grasp the cannula, filled with the CSF tracer, near the tube-covered needle with a pair of curved tweezers held in the dominant hand. Rest the middle finger of the non-dominant hand on the ear bar of the opposite side and hold it steady for the later use as a rest for the cannula.
- Insert the cannula at an angle of 45° relative to the mouse head, passing into the center of the CM, identified by its triangular aspect seen through the dura. Avoid any penetration of the cerebellum or medulla. Ensure that the needle is only inserted to a depth of 1 – 2 mm, i.e. to the point where bevel is entirely under the dura. Release the tweezers holding the cannula, and let the cannula rest on the non-dominant hand.
NOTE: The beveled end of the dental needles will require the application of some force to pierce the dural membrane covering the CM. - If necessary, dry off any CSF leak upon penetration using a surgical eye spear or cotton swabs.
- Drop 2 – 3 drops of cyanoacrylate glue onto the dural membrane surrounding the cannula. Add a drop of glue accelerator to cure the glue immediately. Cover the skull and needle with a mixture of dental cement (approximately 0.5 mg) and cyanoacrylate glue (3 – 5 drops). Immediately after, apply a drop of glue accelerator to cure.
- For chronic cannulation, cut the tubing (leaving approximately 2 – 3 cm attached to the cannula) and seal it with a surgical weld to retain the intracranial pressure (ICP) levels, by preventing CSF leakage through the tubing.
- For chronic cannulation, administer carprofen (5 mg/kg; s.c.)
- For chronic cannulation, place the mouse in a cage, keeping it over a heating pad to maintain body temperature until the animal is fully recovered from anesthesia.
NOTE: The animals should be singly housed in their cages to assure that the cannula remains intact. Ensure that the nose is clear of bedding by placing the mouse on a paper towel or other solid substrate.
NOTE: The following day, when animals are recovered from the surgical procedure performed for cannula insertion, they can be injected with CSF tracers. For injection under anesthesia, administer a mixture of ketamine/xylazine (100 mg/kg; 10 mg/kg, respectively; i.p.) and proceed to steps described in section 2. For injection in awake animals, proceed to section 3.
2. Injection of CSF Tracers via Acute Implanted CM Cannula in Anesthetized Animals
NOTE: For injection of CSF tracers via acute implanted CM cannula in anesthetized animals, immediately after step 8 from previous section, proceed to CSF tracer injection as described below.
- Using a syringe pump, start the injection into the CM of CSF tracers at a rate of 1 μL/min for 5 or 10 min, resulting in a total volume of 5 µL or 10 µL, respectively. At the end of the injection, allow the CSF tracer to circulate throughout the brain for 30 min with the cannula undisturbed.
- After 30 min, cut the tubing connected to the cannula (approximately 4 cm distance from the needle tip) and seal its end using a surgical weld.
- Under deep anesthesia, euthanize the animal by decapitation. Quickly dissect the brain and fix the tissue by immersion in 4% paraformaldehyde (PFA) diluted in phosphate buffered saline (PBS; 0.01M; pH 7.4) overnight (o/n) at 4 °C.
3. Injection of CSF Tracers via Chronically Implanted CM cannula in Awake Animals
- Using a syringe pump, withdraw 7 µL or 12 µl of desired CSF tracer into a cannula composed of approximately 30 cm of PE10 tubing with a beveled dental needle tip of 0.5 cm.
- Gently restrain the animal and cut of approximately 1 cm of the tubing attached to the cannula.
- Still under gentle restraining, quickly connect the cannula filled with CSF tracer to the CM implanted cannula.
- Using a syringe pump, start the injection into the CM of CSF tracers at a rate of 1 µL/min. Inject 7 µL or 12 µL, to achieve a final volume of CSF injected tracer of 5 µL or 10 µL, thus compensating the aCSF remaining in the implanted cannula. At the end of the injection, allow the CSF tracer to circulate throughout the brain for 30 min with the cannula undisturbed. Ensure that the tubing remains attached during injection and circulation periods.
- At the end of CSF tracer circulation time, proceed to euthanasia by decapitation, assuring that the animal is deeply anesthetized. Quickly dissect the brain and fix the tissue by immersion in 4% paraformaldehyde (PFA) diluted in phosphate buffered saline (PBS; 0.01M; pH 7.4) overnight (o/n) at 4 °C.
Representative Results
Upon fixation of mice or rats in a stereotaxic frame, the neck muscles around the occipital crest region are bluntly dissected to expose the cisterna magna (CM). The triangular structure of the CM is readily recognized between the caudal portion of the cerebellum and the medulla (Figure 1A-1C). The cannula is inserted 1 – 2 mm into the CM by gently piercing the atlanto-occipital membrane (Figure 1D). The dura membrane is a tough structure and the insertion of the cannula is improved by tilting the animal head by a 120° in relation to the body. With the aid of an injection pump, differently labeled molecules are then injected into the cisterna magna at controlled rates (Figure 1E). After an interval to allow CSF tracer circulation, animals are euthanized. The brain is carefully dissected and fixed by immersion in 4% PFA o/n at 4 °C. Macroscopic dorsal views of brains harvested from CM-injected rodents show the distribution of CSF tracers in the subarachnoid cisterns of the cerebellum, in the olfactory bulb and in the paravascular space along the middle cerebral arteries (MCAs) (Figure 1F). In the ventral portion of the brain, macroscopic views show CSF tracer distribution along the Circle of Willis (Figure 1G). Histological sections of CM-injected brains further reveal the paravascular distribution of tracers within the brain parenchyma. Mice injected under anesthesia (Figure 1H) (or during natural sleep, see1) show a remarkable increase in tracer distribution into the brain parenchyma in comparison to mice injected while awake and freely moving in their home cage (Figure 1I).
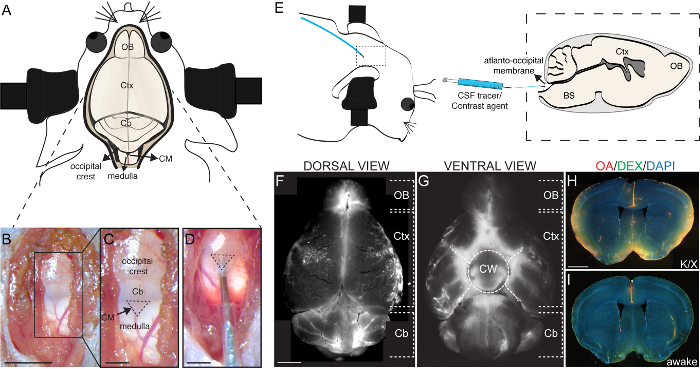
Figure 1: Injection of tracers into the cisterna magna. (A) Schematic overview of the mouse head and brain showing the location of the cisterna magna (CM) in relation to the brain and cranial structures. (B) Photomicrograph of exposed CM after the surrounding neck muscles have been bluntly dissected and pushed to the sides. (C) Higher magnification of the area depicted in B (black rectangle), showing the inverted triangular structure of the CM (dashed line) and its location in relation to the surrounding structures, i.e. occipital crest, cerebellum, and medulla. (D) Photomicrography of the cannula inserted into the CM. (E) Scheme of the lateral view of the mouse fixed head, slightly tilted at an angle of 120° in relation to the body. Inset of dashed rectangle delimited area shows the scheme of a parasagittal view scheme of a parasagittal view of the mouse brain with the cannula inserted into the CM, as outlined in E. A syringe, which is coupled to an injection pump, is used to deliver CSF tracers or contrast agents into the CM through a tube connected to a fine 30G needle. Representative images of a whole mouse brain at 30 min after the end of CM injection with a fluorescent tracer seen from the dorsal (F) and ventral (G) aspects. (H, I,) Representative coronal brain sections counterstained with DAPI (4',6-diamidino-2-phenylindole; 1 µg/mL in PBS) of mice injected with CSF tracers into the CM under anesthetized (H) and wakefulness (I), 30 min after the end of CM injection at a rate of 1 µL/min of a 5 µL volume of a mixture of ovalbumin-AF647 conjugate (OA, 45kDa; 2% in aCSF) and dextran-FITC conjugate (DEX, 3kDa; 2% in aCSF). Scale bars, 5 mm for B, C, F, G; 2 mm for D, and 500 µm for H, I. Cb, cerebellum; CM, cisterna magna; Ctx, cortex; and OB, olfactory bulb. Please click here to view a larger version of this figure.
Discussion
We have presented a protocol that describes a detailed procedure for cisterna magna cannulation (CMc), which offers a straightforward method to deliver labeled molecules to the CSF compartment. CMc allows the subsequent visualization of CSF dynamics, both in vivo and ex vivo, using different imaging modalities or histology.
One of the main advantages of the CMc technique lies in its direct access to the subarachnoid space without the need to expose the brain by craniotomy. By not requiring a cranial window or penetration of the brain parenchyma with a needle tip, CMc allows the delivery of molecules into the CSF compartment and the assessment of the glymphatic system by a minimally invasive procedure, with only brief disturbance of intracranial pressure (ICP).
Notably, the injection into the CM is downstream of the main sources of CSF, the choroid plexi located in the ventricular system (lateral, third and fourth ventricles). From the lateral ventricles, CSF flows to the third ventricle via the intraventricular foramina (the foramen of Monro) and from the third to fourth ventricles via the cerebral aqueduct (the aqueduct of Sylvius) to the brain stem and spinal cord (reviewed in3). CSF reaches the subarachnoid space via the CM by flowing through the median aperture (or the foramen of Magendie), and thus CMc injections bypass the entire ventricular system. However, while this may be problematic in some models of CSF/ISF dynamics through the ventricles, direct injection of tracers into the ventricles requires invasive surgical procedures such as drilling of burr holes in the skull windows, and the application of ventricular injections substantially disrupt the ICP13. Likewise, pressure injection of tracers into the subarachnoid space13,14 in our hands abolishes the flux of CSF tracers along the paravascular space. In contrast, even though CMc entails puncture of the dural membrane, ICP is only transiently perturbed and is quickly restored2.
Using the CMc, glymphatic activity can be measured in anesthetized animals after acute CMc, as well as in awake animals, observing a 24-hour recovery period upon cannula implantation. Acute CMc is suited for combination with 2-photon imaging, which provides detailed information about glymphatic activity within cortex to a depth of approximately 200 µm1,2. Importantly, acute CMc also affords the advantage of supporting unbiased MRI studies, where tracer distribution is followed dynamically, relative to an individual baseline image acquired before the initiation of CSF tracer injection15,16,17. For MRI, the dental needle used for CMc should be replaced by a borosilicate capillary (approximately 1 cm length, tip diameter of approximately 20 µm) attached to the PE tubing.
In contrast to acute cannulation, chronic CMc allows the experimenter to perform CSF tracer injection in animals during natural sleep or under anesthesia, as well as in awake, freely moving animals. This is a crucial factor since glymphatic activity is highly state-dependent; tracer influx to the parenchyma is much greater in animals that were injected under anesthesia or asleep than in animals that were injected in the awake state1. In addition, animals with a chronically implanted cannula can receive CSF tracer in their home cage, thus minimizing confounding factors due to effects of stress and arousal on glymphatic activity. For chronic injections under anesthesia, a mixture of ketamine/xylazine (100 mg/kg; 10 mg/kg, respectively) is recommended. Isoflurane at concentrations above 1.5% induces brain swelling and does not enhance glymphatic activity compared to the awake state1. Note that after CMc implantation, animals should be single housed, in order to assure that CMc implanted animals will not damage the cannula of each other. Also, since the CMc chronic implantation is a recovery surgical procedure, it should be performed under sterile conditions and animals should receive post-operational analgesics.
Importantly, CMc can be used as a method to deliver CSF tracers in mice as well as in rats, with minimal modifications to the protocol. Appropriate anesthetics dose should be administered and the maximum volume of CSF tracer that is injected in rats is 30 µL, due to the differences in the size of the ventricular and subarachnoid spaces between the two species.
Despite its procedural simplicity, some training and practice is required for the experimenter to successfully perform CMc. Since the CM varies in size in between species and individual animals, it is advisable to practice the recognition of its structure. Practicing the procedure using Evans Blue (2% in aCSF) allows the experimenter to confirm correct needle insertion. Occasionally, a vessel will be located directly at the midline of the CM, whereupon the needle should be inserted adjacent to the vessel, but as close as possible to the midline. These cases should be noted, for later confirmation that tracers are evenly distributed, despite the off-center placement of the needle tip. Importantly, the atlanto-occipital membrane covering the CM is mechanically tough, and sufficient pressure should be applied to insert the beveled needle tip. However, it is critical that the pressure applied does not result in plunging the needle tip into the medulla or the cerebellum. To facilitate needle insertion into the CM, the head of animals should be tilted downwards at an angle of 120° relative to the body, which stretches the membrane. Importantly, caution should be taken not to obstruct respiration by this head flexion. If the needle tip should enter the cerebellum, tracers will be retained in the tissue and fail to distribute throughout the subarachnoid space. Damage to the medulla is frequently fatal, whereas cerebellum damage in chronic cannulations can result in prostration and general abnormalities in the behavior of the animals. To minimize the risk of this eventuality, needles with a smaller bevel length can be used.
When moving the muscles in the neck region that covers the dura membrane to insert the cannula into the CM, bleeding can occur. Cotton swabs can be used to absorb the bleeding, but alternatively, ferric chloride solution can be applied. Ferric chloride has a hemostatic effect18, and also triggers the stiffening of neck muscles around the incision site, thus helping to obtain correct insertion of the needle into the CM. Ferric chloride also dries out the skull and dural membrane, presenting better surfaces for adhesion of the cannula. For CMc, apply 1 – 2 drops of ferric chloride solution (10%) (approximately 1 mL) into a cotton swab and dab the neck muscles and the occipital crest. However, topical ferric chloride may possibly seep through the membrane into the CSF, with unknown effects on brain homeostasis. If the use of ferric chloride is a matter of concern, one can instead use wound retractors to keep open the incision site. Careful removal of wound retractors after applying the cyanoacrylate glue avoids inadvertent attachment to the incision site.
CMc is a straightforward and reproducible procedure to deliver molecules directly into the CSF compartment. Since CMc is minimally invasive, it is the preferred method for the visualization of the glymphatic system and can be combined with different imaging modalities such as epifluorescence and 2-photon microscopy or MRI. Thus, CMc represents a great tool for studies of fluid dynamics, namely CSF and ISF, and also of brain fluid clearance. Due to the macroscopic coverage of the glymphatic system, CMc has the potential to be used to deliver molecules brain-wide.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Novo Nordisk Foundation and National Institute of Neurological Disorders and Stroke, NINDS/NIH (M.N.). A.L.R.X. and S.H-R are recipients of a postdoctoral fellowship and a PhD scholarship from the Lundbeck Foundation, respectively.
Materials
| SOPIRA Carpule 30G 0.3 x 12mm | Kulzer | AA001 | |
| Polyethylene Tubing 0.024” OD x 0.011” ID | Scandidact | PE10-CL-500 | |
| 30G x ½” 0.3 x 12 mm Luer-Lock | Chirana T. Injecta | CHINS01 | |
| Chlorhexidine 0.5% (chlorhexidine digluconate) | Meda AS | no catalogue number, see link in comments | http://www.meda.dk/behandlingsomrader/desinfektion/desinfektion-af-hud/klorhexidin-sprit-medic-05/ |
| Alcohol Swab 70% Isopropyl Alcohol 30 x 60mm | Vitrex Medical A/S | 520213 | |
| Viskoese Oejendraeber Ophtha | Ophtha | 145250 | |
| Wooden applicator, Double cotton bud (Ø appr. 4 – 5 mm, length appr. 12 mm) | Heinz Herenz | 1032018 | |
| Eye spears | Medicom | A18005 | |
| Ferric chloride 10% solution | Algeos | NV0382 | |
| Kimtech Science Precision Wipes Tissue Wipers | Kimberly Clark Professional | 05511 | |
| Loctite Super Glue Precision 5g | Loctite | no catalogue number, see link in comments | http://www.loctite-consumer.dk/da/produkter/superglue-liquid.html |
| Insta-Set CA Accelerator | Bob Smith Industries | BSI-152 | |
| Dental Cement Powder | A-M Systems | 525000 | |
| Surgical weld | Kent Scientific Corporation | INS750391 | |
| Hamilton syringe GASTIGHT® , 1700 series, 1710TLL, volume 100 μL, PTFE Luer lock | Hamilton syringes | 1710TLL | |
| LEGATO 130 Syringe pump | KD Scientific | 788130 | |
| Paraformaldehyde powder, 95% | Sigma Aldrich | 158127 | |
| Phosphate buffered saline (PBS; 0.01M; pH 7.4) | Sigma Aldrich | P3813 | |
| Ovalbumin, Alexa Fluor 647 Conjugate | Thermo Fisher Scientific | O34784 | |
DAPI (diamidino-2-phenylindole) Solution (1 mg/mL) |
Thermo Fisher Scientific | 62248 | |
| Dextran, Fluorescein, 3000 MW, Anionic | Thermo Fisher Scientific | D3305 | |
| E-Z Anesthesia EZ-7000 Classic System | E-Z Systems | EZ-7000 | |
| Attane Isofluran 1000 mg/g | ScanVet | 55226 | |
| Euthanimal 200mg/mL (sodium pentobarbital) | ScanVet | 545349 | |
| Ketaminol Vet 100 mg/mL (ketamine) | Intervet International BV | 511519 | |
| Rompin Vet 20 mg/mL (xylazin) | KVP Pharma + Veterinär Produkte GmbH | 148999 | |
| Xylocain 20 mg/mL (lidocain) | AstraZeneca | 158543 | |
| Marcain 2.5 mg/mL (bupivacain) | AstraZeneca | 123918 | |
| Bupaq Vet 0.3 mg/mL (buprenorphine) | Richter Pharma AG | 185159 |
References
- Xie, L., et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. , 373-377 (2013).
- Iliff, J. J., et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
- Jessen, N. A., Munk, A. S. F., Lundgaard, I., Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 40, 2583-2599 (2015).
- Louveau, A., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. , (2015).
- Aspelund, A., et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991-999 (2015).
- Kress, B. T., et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845-861 (2014).
- Plog, B. A., et al. Biomarkers of Traumatic Injury Are Transported from Brain to Blood via the Glymphatic System. J. Neurosci. 35, 518-526 (2015).
- Jiang, Q., et al. Impairment of glymphatic system after diabetes. J. Cereb. Blood Flow Metab. , (2016).
- Peng, W., et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 93, 215-225 (2016).
- Orešković, D., Klarica, M. The formation of cerebrospinal fluid: Nearly a hundred years of interpretations and misinterpretations. Brain Res. Rev. 64, 241-262 (2010).
- Dusart, I., Schwab, M. E. Secondary Cell Death and the Inflammatory Reaction After Dorsal Hemisection of the Rat Spinal Cord. Eur. J. Neurosci. 6, 712-724 (1994).
- Eide, K., Eidsvaag, V. A., Nagelhus, E. A., Hansson, H. -. A. Cortical astrogliosis and increased perivascular aquaporin-4 in idiopathic intracranial hypertension. Brain Res. , (2016).
- Pullen, R. G., DePasquale, M., Cserr, H. F. Bulk flow of cerebrospinal fluid into brain in response to acute hyperosmolality. Am. J. Physiol. 253, F538-F545 (1987).
- Ichimura, T., Fraser, P. A., Cserr, H. F. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 545, 103-113 (1991).
- Iliff, J. J., et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Invest. 123, 1299-1309 (2013).
- Ratner, V., et al. Optimal-mass-transfer-based estimation of glymphatic transport in living brain. Proc. SPIE–the Int. Soc. Opt. Eng. 9413, (2015).
- Lee, H., et al. The Effect of Body Posture on Brain Glymphatic Transport. J. Neurosci. 35, 11034-11044 (2015).
- Nouri, S., Sharif, M. R., Sahba, S. The effect of ferric chloride on superficial bleeding. Trauma Mon. 20, e18042 (2015).

