Dynamic Electrochemical Measurement of Chloride Ions
Summary
Dynamic measurement of chloride ions is presented. Transition time of an Ag/AgCl electrode, during a chronopotentiometric technique, can give the concentration of chloride ions in electrolyte. This method does not require a stable conventional reference electrode.
Abstract
This protocol describes the dynamic measurement of chloride ions using the transition time of a silver silver chloride (Ag/AgCl) electrode. Silver silver chloride electrode is used extensively for potentiometric measurement of chloride ions concentration in electrolyte. In this measurement, long-term and continuous monitoring is limited due to the inherent drift and the requirement of a stable reference electrode. We utilized the chronopotentiometric approach to minimize drift and avoid the use of a conventional reference electrode. A galvanostatic pulse is applied to an Ag/AgCl electrode which initiates a faradic reaction depleting the Clˉ ions near the electrode surface. The transition time, which is the time to completely deplete the ions near the electrode surface, is a function of the ion concentration, given by the Nernst equation. The square root of the transition time is in linear relation to the chloride ion concentration. Drift of the response over two weeks is negligible (59 µM/day) when measuring 1 mM [Clˉ]using a current pulse of 10 Am-2. This is a dynamic measurement where the moment of transition time determines the response and thus is independent of the absolute potential. Any metal wire can be used as a pseudo-reference electrode, making this approach feasible for long-term measurement inside concrete structures.
Introduction
A chloride ion sensor based on the transition time measurement of an Ag/AgCl electrode is presented. The aim is to avoid the inherent drifts during the long-term continuous monitoring of the chloride ions in the electrolyte. Chronopotentiometric measurement, which is a dynamic measurement approach, of an Ag/AgCl electrode is used for this purpose. Here a rate of change of potential of an Ag/AgCl electrode is measured during a stimulus (galvanostatic pulse). The advantage of this approach is demonstrated by eluding the liquid-junction reference electrode and instead using any metal wire as a pseudo-reference electrode, therefore allowing the detection of Clˉ ions concentration for long-term (years) and in situ applications, such as measurement inside concrete structures.
Chloride ions in concrete structures is one of the major causes of degradation1,2. It initiates pitting corrosion in the reinforcement steel and results in the ultimate failure of the structure3. Therefore, measuring Clˉ ions in concrete is inevitable to predict the service life and maintenance cycle of a structure4,5. Different sensing principles have been reported for chloride ion measurement in concrete such as electrochemical6,7, optical8,9 and electromagnetic10,11. However, optical and electromagnetic methods have bulky setups, are difficult to integrate as a stand-alone system and have issues with selectivity12. In electrochemical technique, potentiometric measurement of an Ag/AgCl electrode is the state of the art approach6,7,13. Despite promising results, this approach is limited to lab-scale measurement since the drifts in reference potential and diffusion potential drop results in flawed data14,15. A transition time approach based on the dynamic electrochemical measurement (DEM) could alleviate the problem due to potential drift16.
In the DEM, a system's response to an applied stimulus is measured17-19. The example of such a system is chronopotentiometry. Here an applied current pulse is used as a stimulus depleting ions near the electrode surface and the corresponding potential response is measured. An anodic current at an Ag/AgCl electrode initiates a faradaic reaction (Ag + Clˉ 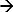 AgCl + eˉ) resulting in a depletion of Clˉ ions near electrode surface. The potential change is a function of the applied current and the concentration of the (selective) ions in the electrolyte12,20. The moment these ions deplete completely near the electrode surface the rate of change of potential rises rapidly, giving an inflection point21. The inflection point on the potential-time response curve (chronopotentiogram) shows the transition time and can be determined from the maximum of the first derivative of the potential response22. The transition time is a characteristic of the ion concentration. This approach has been used to determine different ions concentration17 and the pH of electrolytes23,24. In case of an Ag/AgCl electrode as a working electrode (to which current is applied) the depleting ions will be chloride ions17. Therefore measuring its transition time will determine its concentration.
AgCl + eˉ) resulting in a depletion of Clˉ ions near electrode surface. The potential change is a function of the applied current and the concentration of the (selective) ions in the electrolyte12,20. The moment these ions deplete completely near the electrode surface the rate of change of potential rises rapidly, giving an inflection point21. The inflection point on the potential-time response curve (chronopotentiogram) shows the transition time and can be determined from the maximum of the first derivative of the potential response22. The transition time is a characteristic of the ion concentration. This approach has been used to determine different ions concentration17 and the pH of electrolytes23,24. In case of an Ag/AgCl electrode as a working electrode (to which current is applied) the depleting ions will be chloride ions17. Therefore measuring its transition time will determine its concentration.
Protocol
1. Chip Fabrication
Note: The chip consists of an Ag/AgCl working electrode (WE), an Ag/AgCl pseudo-reference electrode (pseudo-RE) and a platinum counter electrode on a glass chip. The silver metal is deposited on a glass chip, using standard cleanroom processes16. It is then chloridized in 0.1 M FeCl3 solution for 30 sec to form an AgCl layer over the surface. The Ag/AgCl WE (area = 9.812 mm2) is located in the center, surrounded by the Ag/AgCl pseudo-RE as shown in Figure 1.
- Deposit the silver metal on glass to form a planar silver electrode. The step-by-step chip fabrication in a cleanroom is given as follows:
- Lithography
- For wafer cleaning, immerse the glass wafer first into conc. HNO3 bath to remove any organic contamination and then into 1 M KOH to clean the surface.
- Apply a positive photo resist (200 nm thick) to the wafer by spin coating and prebake the resist at 95 °C for 15 min.
- Expose the wafer to UV light for 5 sec through a glass mask.
- Develop the photoresist in developer solution. This will expose the area of glass where electrodes will be deposited and the remaining area will be covered by the unexposed resist.
- Glass Etching
- Immerse the developed glass wafer in the KOH bath to etch the exposed area of the glass.
- Metal Deposition (Sputtering)
- Deposit silver metal on the exposed glass area.
- First deposit a 20 nm titanium (Ti) layer (by sputtering) as an adhesion layer for glass.
- Then deposit 70 nm palladium (Pd) layer (by sputtering) as a diffusion barrier between silver and Ti.
- Finally deposit 500 nm of silver metal. This is the thickness of the deposited metal. The area of the working electrode is 4 mm2.
- Remove the remaining resist by lift-off process in an acetone bath.
- Dice the wafer into the single chip size. The chip is now ready to be chloridized to form an on-chip Ag/AgCl electrode.
- Deposit silver metal on the exposed glass area.
- Lithography
- Place the chip in an in-house designed Polytetrafluoroethylene chip holder, as shown in Figure 1. The holder provides easy electrical connections via push-pins (or spring-loaded pins) and contains an electrochemical cell.
- Chloridize the silver electrode to form an Ag/AgCl electrode on the chip. For that, pour 0.1 M FeCl3 solution in the cell for 30 sec. or till the silver color of the electrode becomes dark grayish. Longer immersion is not critical whereas shorter immersion time can lead to no deposition of Ag/AgCl.
- Rinse the chip with deionized water to clean the remaining FeCl3.The chip is now ready to be used for the chronopotentiometric measurement.
2. Electrolyte Preparation
Note: The electrolytes were prepared with different concentration of potassium chloride in 0.5 M KNO3.
- Prepare 50 ml of 50 mM KCl electrolyte stock solution (0.186 g of KCl in 50 ml of solution).
- In the same solution add 2.52 g of KNO3 to form a 0.5 M KNO3 background electrolyte.
- Keep the starting concentration of chloride to zero and KNO3 to 0.5 M. In other words add 2.52 g of KNO3 in a 50 ml of deionized water.
- Add 50 mM KCl to increase the chloride ion concentration (from 1 mM to 6 mM) of the electrolyte, systematically, as shown in the following steps.
- Add 100 µl of the 50 mM KCl into a 4.9 ml of 0.5 M KNO3 to get 1 mM [Clˉ].
- Add 104 µl of the 50 mM KCl solution into the solution of step 2.4.1 to get 2 mM [Clˉ].
- Add 108.41 µl of the 50 mM KCl solution into the solution of step 2.4.2 to get 3 mM [Clˉ].
- Add 113.19 µl of the 50 mM KCl solution into the solution of step 2.4.3 to get 4 mM [Clˉ].
- Add 118.2 µl of the 50 mM KCl solution into the solution of step 2.4.4 to get 5 mM [Clˉ].
- Add 123.3 µl of the 50 mM KCl solution into the solution of step 2.4.5 to get 6 mM [Clˉ].
3. Experimental Setup
Note: The measurements were performed using a potentiostat (Biologic Science Instruments, France).
- Place the chip in the chip holder and connect the corresponding terminal of the potentiostat.
- Connect the working electrode terminal of the potentiostat to the Ag/AgCl electrode.
- Connect the reference electrode terminal of the potentiostat to another Ag/AgCl electrode.
- Connect the counter electrode terminal of the potentiostat to the platinum electrode.
- Place the complete experiment setup in a faraday cage to avoid ambient noise.
4. Instrument Operational Settings
Note: The operational parameters of the electrochemical cell are controlled via the user interface of the potentiostat. The potential response of the Ag/AgCl WE during an applied current pulse is measured for different concentrations of chloride ion.
- In the potentiostat program, open the technique chronopotentiometry and set the operational parameters.
- Set the following parameter according to the requirements:
- Current (Is)
- Time of applied current (ts)
- Start this technique by clicking the play button in the potentiostat program.
Note: The potentiostat program displays the potential response as a function of time under the applied current pulse.
- Set the following parameter according to the requirements:
5. Measurements and Data Analysis
- Draw the calibration curve of the transition time vs. Clˉ ion concentration in electrolyte.
- Change the Clˉ concentration in the KCl electrolyte, systematically.
- Start the measurement with 5 ml of 1 mM KCl with 0.5 M KNO3 background electrolyte in the electrochemical cell.
- Start the chronopotentiometric experiment by using the potentiostat and apply a current of 10 mA/cm-2 for 10 sec and store the data.
- Systematically change the concentration to 6 mM with 1 mM increments and repeat the measurements.
- Store the measured data as a.mpt file and analyze the data file in data processing program.
- Analyze the data using an in-house developed data processing program to calculate the first derivative of the potential response and calculate the peak of the first derivative. The peak of the first derivative is the transition time.
Note: The complete coding of data processing program is given in the supplementary information of Abbas et al., 2014.
- Analyze the data using an in-house developed data processing program to calculate the first derivative of the potential response and calculate the peak of the first derivative. The peak of the first derivative is the transition time.
- Repeat the measurement three times with the interval of 1 hr each.
- Open all the data files in data processing program and calculate the transition time for each measurement. Detail steps of transition time calculation is given below:
- Plot the potential difference vs. time obtained from the data files of chronopotentiometric measurement.
- Calculate the first derivative of the potential response.
- Indicate the maximum of the first derivative and the time of it. The time of the maximum of first derivative is the transition time.
- For the calibration curve, plot the square root of the transition time with respect to the concentration of Clˉ ions.
- Along the measured data plot the theoretical curve based on the Sand equation25. Calculate back the diffusion coefficient from the data plot.
- In the measured data plot between transition time and the [Clˉ] take any point on the line and record the value of transition time and the [Clˉ]. The Sand equation is given as:
τ = (FC*/2j(1-tCl–))2 Dπ
Here, C* is the bulk Clˉ ion concentration, F is the Faraday constant, j is the current density, tCl– is the transport number and D is the diffusion coefficient. Neglect the tCl– since it approaches zero for higher background electrolyte concentration. - The only remaining variable in the Sand equation in the diffusion coefficient; put the value of transition time, the chloride ion concentration and the applied current in the Sand equation getting the value of the diffusion coefficient.
- In the measured data plot between transition time and the [Clˉ] take any point on the line and record the value of transition time and the [Clˉ]. The Sand equation is given as:
- Change the Clˉ concentration in the KCl electrolyte, systematically.
- Drift Measurement
- Pour 5 ml of 1 mM of the KCl in the electrochemical cell.
- In the potentiostat, set the applied current to be 10 Am-2 and time to be 10 sec.
- Measure the potential response for 2 weeks with three measurement each day with an interval of 3 hr between measurements.
- Refresh the electrolyte, i.e., 1 mM KCl, every day before performing the measurements.
- Plot the transition time over two weeks of measurements. The change in the transition time per day is the drift of the transition time response.
- Effect of the pseudo-reference electrode on the transition time measurements
Note: Various pseudo-references such as a Ag/AgCl wire, a platinum wire and a steel wire are tested to measure the transition time.- Pour 4 mM of KCl electrolyte in the electrochemical cell.
- Use Ag/AgCl as a pseudo-reference electrode and connect it to the reference electrode terminal of the potentiostat. Due to anodic current pulse the Clˉ ions are depleted near the Ag/AgCl working electrode.
- Perform the chronopotentiometric measurement by applying a current density of 15 Am-2 for 10 sec.
- Repeat the measurements with platinum as a pseudo-reference electrode and record the data.
- Repeat the measurements with steel bar as a pseudo-reference electrode and record the data.
- Plot the measured transition time for various pseudo-reference electrodes used.
- Repeat the complete set of measurement with 5 mM of KCl and plot the transition time using various pseudo-reference electrodes.
Representative Results
The Ag/AgCl electrode is fabricated on a glass chip (Figure1) using a standard cleanroom process. The chronopotentiometric measurement setup (Figure 2) was used and response was measured using a potentiostat. To observe the effect of Clˉ ion concentration on the transition time, solutions containing 4, 5 and 6 mM of Clˉ ions in a 0.5 M KNO3 background are measured (Figure 3). The calibration curve of the square root of the transition time versus Clˉ ion concentration is plotted along with theoretical curve (Figure 4). Transition time response was measured for two weeks to evaluate the drift in the measurement (Figure 5). Transition time is measured in 4 and 5 mM Clˉ ion concentration for different pseudo-reference electrode to observe the effect of reference system on transition time (Figure 6). The range of current density over the range of 1-6 mM [Clˉ] is evaluated from the Sand equation (Table 1).
The transition time was measured in various concentrations of Clˉ ions, namely 4, 5 and 6 mM, as shown in Figure 3. The corresponding potential response is visible along with its first derivative. The peaks of the first derivatives give the transition times. The time instant of the peak is shifting to higher values with the increase in [Clˉ]. This is expected as more Clˉ ions present in the bulk electrolyte means it will take longer to completely deplete the Clˉ ions near the WE surface. The measured transition times for mentioned concentrations are 2.69, 4.28 and 5.92 sec, respectively.
The transition time measurements were calibrated against the known concentration of chloride ions. The measured data and its linear fit is presented in Figure 4. The square root of the transition time is in linear correlation to the [Clˉ], as predicted by the Sand equation. The apparent diffusion coefficient of Clˉ ions from the measured data is found to be 2.280 x 10-9 m2sec-1, which is in good agreement with the theoretical value, D = 2 x 10-9 m2sec-1. The deviation can be due to the uncertainty in the current density value attributed to the changing surface area during the current pulse. Furthermore the deviation is relatively larger for higher concentrations, e.g., 7 mM. This is attributed to the relatively large thickness of the concentration profile, which makes it more sensitive to convection.
The dynamic nature of transition time ensures a drift free measurement. To analyze the drift, the transition time for 1 mM Clˉ was measured over two weeks, as shown in Figure 5. There is a decreasing trend in transition time, 0.23 msec/day (corresponds to 0.8 µM/day), in the linear fit of the data. Nonetheless, this change is small and could be attributed to handling errors, changing Clˉ ion concentration due to evaporation, change in apparent current density and temperature variation over the measurement period. It is therefore difficult to give a concrete conclusion about the drift of the sensor; either there is no inherent drift or the drift is relatively small.
Another aspect of the dynamic nature of transition time measurement is its independence on a reference system. Various pseudo-references (an Ag/AgCl wire, a platinum wire and a steel wire) were set up to measure the transition time of an Ag/AgCl electrode in 4 and 5 mM [Clˉ]. The measurements are shown in Figure 6. For various pseudo-references the response does not change significantly, transition time varies within 80 msec (± 75 µM at 4 and 5 mM Clˉ ions). Therefore the reference system has no systematic effect; any metal wire can be a used as a pseudo-reference electrode for transition time measurements.
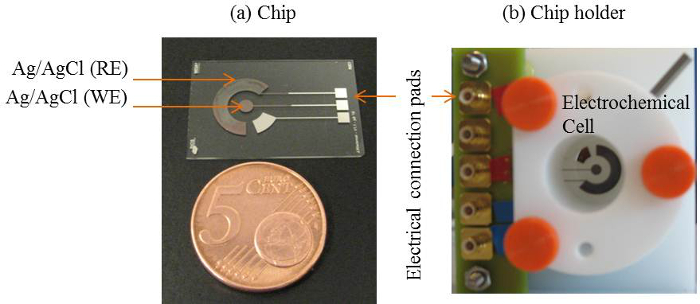
Figure 1. Chloride sensor chip along with the electrochemical cell. (A) The Clˉ ion sensor chip with Ag/AgCl electrodes on a glass substrate and electrical connection pads. The dimension of the glass chip is 15 x 20 mm2 whereas the exposed area in the electrochemical cell is 78 mm2. (B) The complete electrochemical cell (chip holder) with the mounted chip. The middle circular area shows the area of the chip exposed to the electrolyte16. Please click here to download this file.
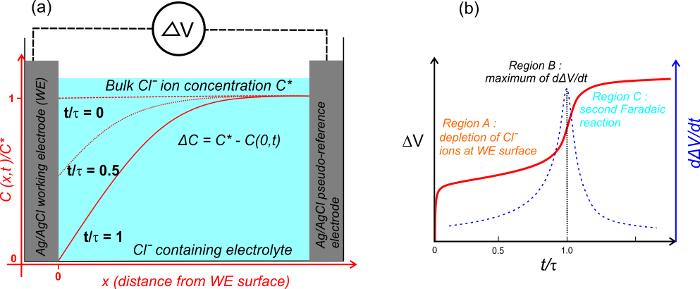
Figure 2. Schematic of transition time approach. (A) Schematic of the Clˉ ion detection approach. During an applied current pulse at the WE with respect to a CE (not included in this Figure), chloride ions deplete at the Ag/AgCl WE, resulting in the illustrated Clˉ ion concentration profile. (B) Schematic of the ΔV and dΔV/dt response. The solid line (-) and dashed line (–) represent ΔV and dΔV/dt, respectively. τ is the transition time and t is the duration of the applied current pulse. Here, t/τ is the ratio of the applied current time and the transition time when t/τ = 1, the Clˉ ions at the surface of the working electrode depletes completely16. Please click here to download this file.
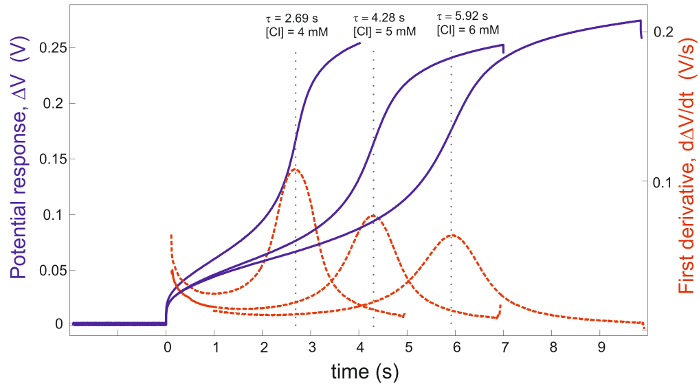
Figure 3. Transition time measurements. The chronopotentiograms (solid line) and their first derivatives (dashed line) of the Ag/AgCl electrode with respect to another Ag/AgCl pseudo-RE electrode in solutions of 4, 5 and 6 mM Clˉ ions. The background electrolyte is 0.5 M KNO3, the applied current pulse is 10 Am-2 and the ambient temperature is 20.9 °C. The maximum slopes of the potential responses are indicated by the dotted lines16. Please click here to download this file.
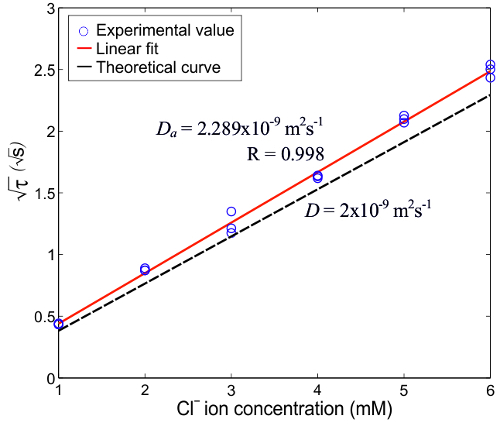
Figure 4. Calibration curve. A Calibration curve showing the square root of the transition time versus the Clˉ ion concentration, in a 0.5 M KNO3 background electrolyte. The circular points (o) are the measured data and the solid line (-) is the linear fit. The dashed line (–) is the theoretical curve from the Sand equation, eq. (2). The applied current pulse is 10 Am-2 and the ambient temperature is 20.8 °C. D and Da are the theoretical and apparent (measured) diffusion coefficients, respectively16. Please click here to download this file.
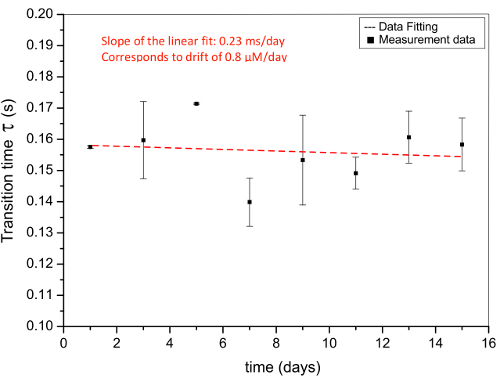
Figure 5. Drift analysis. Subsequent measurements of the transition time over two weeks. Each measurement was carried out every other day, with 3 measurements performed each day and 3 hr between each same-day measurement. The electrolyte contains 1 mM of Clˉ ions in a 0.5 M KNO3 background and the applied current pulse is 10 Am-2. The data points and linear fit are denoted by (+) and a dashed line, respectively16. Please click here to download this file.
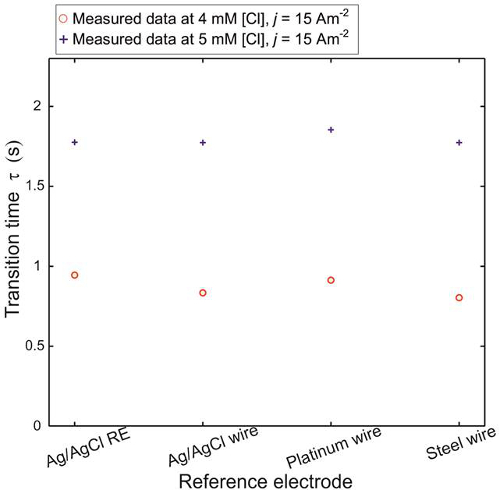
Figure 6. Effect of different pseudo-reference electrodes. Measured transition times using pseudo-REs composed of a Ag/AgCl liquid junction RE, Ag/AgCl wire, platinum wire and steel wire. The applied current pulse is 15 Am-2 and the ambient temperature is 21.2 °C. Here, + and o marks show the measured data for 4 and 5 mM of Clˉ ions in a 0.5 M KNO3 background, respectively16. Please click here to download this file.
| [Clˉ] range (mM) |
Current density, j (Am-2) |
(j/C*)min (A∙m∙mol-1) |
(j/C*)max (A∙m∙mol-1) |
τmin (s) |
τmax (s) |
| 1 to 6 | 10 | 1.66 | 10 | 0.146 | 5.26 |
Table 1. The selected current density for a Clˉ ion concentration range of 1 to 6 mM, evaluated from eq. (3). The corresponding values of the ratio j/C and transition time are also given16.
Discussion
The transition time is the moment of inflection; it is theoretically independent of the reference potential i.e., the reference electrode. Therefore any metal wire can be used as a pseudo-reference electrode for transition time measurements. In contrast to the existing potentiometric measurement of chloride ions in concrete this method enables a long-term and calibration free measurement. Furthermore the sensitivity and the detection range of concentration can be tuned by adjusting the applied current pulse. For higher Clˉ concentrations, which are the case in concrete, higher current pulse should be applied to keep the transition time within 6 sec.
Although the dynamic measurement using the transition time of an Ag/AgCl electrode seems an attractive alternative for reference and calibration free detection of Clˉ ions. The interference from other anions, such as hydroxides and halides, can limit it feasibility especially when the concentration of interfering ions is higher than the Clˉ ions. For example a mixed (broaden) peak of transition time is observed if the [OHˉ] is 10 times higher than the [Clˉ] in the electrolyte. In this case the transition time peaks for OHˉ and Clˉ ions cannot be differentiated. The detection range of [Clˉ] depends upon the concentration of interfering ions such as OHˉ ions7. We have tested down to 1 µM KCl in the absence of interfering ions. Moreover, in the presence of iodide (Iˉ) and bromide (Brˉ) ions the sensor will preferentially detect the transition time for Iˉ and Brˉ ions rather than chloride. Typically in concrete interference from Iˉ and Brˉ ions is irrelevant due to their absence or negligible amount. Besides, interference from hydroxide and halides ions can be compensated by covering the Ag/AgCl electrode with chloride ion-selective-polymer membrane (ionophores)26.
The applied current pulse is a critical parameter in this determining the transition time and should be carefully selected. Higher current pulse can rapidly degrade the AgCl surface with the formation of silver oxide. Whereas lower current pulse results in a longer transition time, consequently inducing errors in transition time due to undesired convection. The current pulse should be adjusted in such a way that the transition time is within 6 sec. This can be evaluated by putting transition time value and concentration limits in the Sand equation16. The surface degradation due to higher current pulses can be remedied by applying a reverse current (cathodic charge, Ic x tc) after each measurements (anodic charge, Ia x ta). The cathodic current will remove the excess AgCl deposited during the anodic current pulse. The cathodic current should be lower in amplitude and longer in time, tc, such that the charge of anodic and cathodic current should be equal, i.e., Ia ta = Ic tc. Furthermore, a rough range of the [Clˉ] ion concentration should be determined in advance and the current pulse is applied in such a way that the transition time should reach within 5 to 10 sec. Furthermore, the potentiostat program should be adjusted such that the anodic current stops once the transition time is reached. This will avoid the formation of AgO.
This method can be used for long term measurement of Clˉ ions in concrete giving long-term and reliable measurements. It can be used to monitor Clˉ ions in in drinking water, swimming pools and biological samples. Furthermore, it can be extended to monitor other halide ions like iodide and bromide in drinking water.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is a part of the STW project “Integral solution for sustainable construction (IS2C, Fleur van Rossem for her support during the chip fabrication, Justyna Wiedemair for the chip design and Allison Bidulock for her support during the manuscript preparation.
Materials
| Platinum wire (≥99.99% trace metals) | Sigma Aldrich, the Netherlands | EP1330-1EA | |
| Potassium chloride (BioXtra, ≥99.0%) | Sigma Aldrich, the Netherlands | P9333-500G | |
| Potassium hydroxide (90% pure reagent grade) | Sigma Aldrich, the Netherlands | 484016-1KG | |
| Ferric chloride | Sigma Aldrich, the Netherlands | 451649-1G | |
| potassium nitrate (> 99% reagent grade) | Sigma Aldrich, the Netherlands | P6083-500G | |
| Ag/AgCl liquid junction reference electrode | BASi, USA | model MF-2079 | |
| VSP potentiostat | Biologic Science Instruments, France | VSP 300 | |
| Steel wire | Microlab TU Delft | ||
| Silver wire | Sigma Aldrich, the Netherlands |
References
- Page, C., Treadaway, K. Aspects of the electrochemistry of steel in concrete. Nature. 297, 109-115 (1982).
- Koleva, D. A., Hu, J., van Breugel, K., Boshkov, N., de Wit, H. Conventional and pulse cathodic protection of reinforced concrete: electrochemical approach and microstructural investigations. ECS Transactions. 1, 287-298 (2006).
- Montemor, M., Simoes, A., Ferreira, M. Chloride-induced corrosion on reinforcing steel: from the fundamentals to the monitoring techniques. Cement and Concrete Composites. 25, 491-502 (2003).
- Wegen, G., Polder, R. B., Breugel, K. V. Guideline for service life design of structural concrete: A performance based approach with regard to chloride induced corrosion. Heron. 57 (3), (2012).
- Yoon, I., Koenders, E. Theoretical time evolution of critical chloride content in concrete. Structural Durability & Health Monitoring. 5, 275-294 (2010).
- Du, R. G., Hu, R. G., Huang, R. S., Lin, C. J. In situ measurement of Cl-concentrations and pH at the reinforcing steel/concrete interface by combination sensors. Analytical Chemistry. 78, 3179-3185 (2006).
- Angst, U., Elsener, B., Larsen, C. K., Vennesland, &. #. 2. 1. 6. ;. Potentiometric determination of the chloride ion activity in cement based materials. Journal of Applied Electrochemistry. 40, 561-573 (2010).
- Laferrière, F., Inaudi, D., Kronenberg, P., Smith, I. F. A new system for early chloride detection in concrete. Smart Materials and Structures. 17, 045017 (2008).
- Tang, J. L., Wang, J. N. Measurement of chloride-ion concentration with long-period grating technology. Smart Materials and Structures. 16, 665 (2007).
- Kohri, M., Ueda, T., Mizuguchi, H. Application of a near-infrared spectroscopic technique to estimate the chloride ion content in mortar deteriorated by chloride attack and carbonation. Journal of Advanced Concrete Technology. 8, 15-25 (2010).
- Tripathi, S. R., Inoue, H., Hasegawa, T., Kawase, K. Non-destructive Inspection of Chloride Ion in Concrete Structures Using Attenuated Total Reflection of Millimeter Waves. Journal of Infrared, Millimeter, and Terahertz Waves. 34, 181-186 (2013).
- Abbas, Y., Olthuis, W., van den Berg, A. A chronopotentiometric approach for measuring chloride ion concentration. Sensors and Actuators B: Chemical. 188, 433-439 (2013).
- Climent-Llorca, M. A., Viqueira-Pérez, E., Lòpez-Atalaya, M. M. Embeddable Ag/AgCl sensors for in-situ monitoring chloride contents in concrete. Cement and Concrete Research. 26, 1157-1161 (1996).
- Myrdal, R. . The electrochemistry and characteristics of embeddable reference electrodes for concrete. , (2014).
- Angst, U., Vennesland, &. #. 2. 1. 6. ;., Myrdal, R. Diffusion potentials as source of error in electrochemical measurements in concrete. Materials and Structures. 42, 365-375 (2009).
- Abbas, Y., de Graaf, D. B., Olthuis, W., van den Berg, A. No more conventional reference electrode: Transition time for determining chloride ion concentration. Analytica Chimica Acta. 821, 81-88 (2014).
- Meyer, R. E., Posey, F. A., Lantz, P. M. Chronopotentiometry of the Ag− AgCl system and analysis for the chloride ion. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 19, 99-109 (1968).
- Olthuis, W., Langereis, G., Bergveld, P. The metrits of differential measuring in time and space. Biocybernetics and Biomedical Engineering. 21, 5-26 (2001).
- Bakker, E., Bhakthavatsalam, V., Gemene, K. L. Beyond potentiometry: robust electrochemical ion sensor concepts in view of remote chemical sensing. Talanta. 75, 629-635 (2008).
- Olthuis, W., Bomer, J., Bergveld, P., Bos, M., Van der Linden, W. Iridium oxide as actuator material for the ISFET-based sensor-actuator system. Sensors and Actuators B: Chemical. 5, 47-52 (1991).
- Bergveld, P., Eijkel, J., Olthuis, W. Detection of protein concentrations with chronopotentiometry. Biosensors and Bioelectronics. 12, 905-916 (1997).
- Iwamoto, R. Derivative chronopotentiometry. Analytical Chemistry. 31, 1062-1065 (1959).
- Olthuis, W., Bergveld, P. Simplified design of the coulometric sensor-actuator system by the application of a time-dependent actuator current. Sensors and Actuators B: Chemical. 7, 479-483 (1992).
- Olthuis, W., Bergveld, P. Integrated coulometric sensor-actuator devices. Microchimica Acta. 121, 191-223 (1995).
- Bard, A. J., Faulkner, L. R. . Electrochemical methods: fundamentals and applications. , (2001).
- Bakker, E., Bühlmann, P., Pretsch, E. Polymer Membrane Ion-Selective Electrodes-What are the Limits? . Electroanalysis. 11, 915-933 (1999).

