成像膜电位与基因编码荧光电压传感器的两种类型
Summary
A method for imaging changes in membrane potential using genetically encoded voltage indicators is described.
Abstract
Genetically encoded voltage indicators (GEVIs) have improved to the point where they are beginning to be useful for in vivo recordings. While the ultimate goal is to image neuronal activity in vivo, one must be able to image activity of a single cell to ensure successful in vivo preparations. This procedure will describe how to image membrane potential in a single cell to provide a foundation to eventually image in vivo. Here we describe methods for imaging GEVIs consisting of a voltage-sensing domain fused to either a single fluorescent protein (FP) or two fluorescent proteins capable of Förster resonance energy transfer (FRET) in vitro. Using an image splitter enables the projection of images created by two different wavelengths onto the same charge-coupled device (CCD) camera simultaneously. The image splitter positions a second filter cube in the light path. This second filter cube consists of a dichroic and two emission filters to separate the donor and acceptor fluorescent wavelengths depending on the FPs of the GEVI. This setup enables the simultaneous recording of both the acceptor and donor fluorescent partners while the membrane potential is manipulated via whole cell patch clamp configuration. When using a GEVI consisting of a single FP, the second filter cube can be removed allowing the mirrors in the image splitter to project a single image onto the CCD camera.
Introduction
本文的主要重点是说明使用基因编码荧光蛋白在体外膜电位的变化的光学成像系统。在膜电位成像变化提供学习的神经元回路的活性的令人兴奋的可能性。当改变膜电位结果在荧光强度变化,相机的各像素变为一个替代电极使神经元活动的非侵入式测量。四十多年来,有机电压敏感的染料已经观察膜电位1-4的变动情况。然而,这些染料缺乏蜂窝特异性。此外,某些类型的细胞都难以染色。通过使细胞中专门研究表达荧光电压敏感探测器遗传编码电压指示器(GEVIs)克服这些限制。
有三类GEVIs的。第一类GEVI的使用VOltage感应来自电压传感磷酸酶区带是单一荧光蛋白(FP)5-9或荧光共振能量转移(FRET)对10-12。第二类传感器的使用微生物的视紫红质作为荧光指示剂直接13-15或通过电致变色FRET 16,17。第三类利用两个组件,该遗传成分是膜锚定的FP和第二组分是一种膜结合的淬灭染料18-20。而第二和第三类是用于在体外和切片实验19,20有用,只有第一类传感器是目前用于体内分析6是有用的。
在本报告中,我们将在体外使用第一类GEVIs的(图1)表明膜电位的摄像。该第一类电压传感器的是最简单的转换到体内成像 。由于GEVIsütilizing融合于FP的电压传感域中大约50倍比视紫红质类传感器的更亮,它们可以通过使用弧光灯照明,而不是要求一个非常强大的激光进行成像。亮度的差异的另一个后果是,第一类GEVIs的很容易超过大脑的自体荧光。基于视紫红质探针不能。第三类传感器的只是为第一类是光明,但需要添加化学淬灭剂这是难以在体内的管理。
我们将因此表现出探针与单个FP(Bongwoori)8和由FRET对的探针(纳比2)12采集。该FRET本报告中构建的VSFP-CR(电压敏感荧光蛋白-四叶草mRuby2)的蝴蝶版本11组成的绿色荧光供体,三叶草,和红色荧光受体,mRuby2,名为彩蝶2.242和彩蝶2.244 <s达> 12。引进这些类型的录音应该给研究人员更好地了解信息GEVIs的类型可以提供。
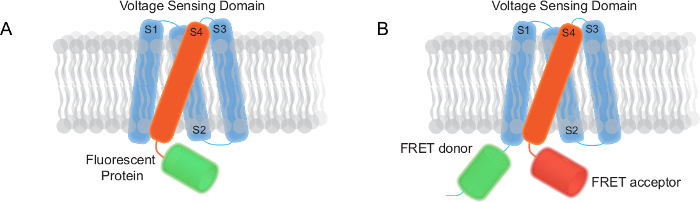
图1:在本报告中 (A) 成像基因编码的电压指标(GEVIs)基于GEVI有一个跨膜电压感应域和荧光蛋白单FP 的两种类型 。 (B)基于FRET GEVI由跨膜电压感应域,FRET供体和受体的。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
神经系统采用几种不同的方式的电压,抑制导致轻微的超极化,突触输入导致轻微的去极化和动作电位产生一个比较大的电压变化。通过GEVIs测量膜电位变化的能力提供了同时分析神经回路中的几个组件的有前途的潜力。在这份报告中,我们展示了使用GEVIs膜电位影像学改变的根本方法。
用于成像的变化电压的主要关键是GEVI在质膜的有效表达。胞内表达创建降低了探头的SNR一…
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the World Class Institute (WCI) program of the National Research Foundation of Korea funded by Ministry of Education, Science, and Technology of Korea Grant WCI 2009-003 and Korea Institute of Science and Technology Institutional Program Project 2E24210. Sungmoo Lee was supported by Global Ph.D. Fellowship program (NRF-2013H1A2A1033344) of the National Research Foundation (NRF) under the Ministry of Education (MOE, Korea).
Materials
| Inverted Microscope | Olympus | IX71 | |
| 60X objective lens (numerical aperture = 1.35) | Olympus | UPLSAPO 60XO | |
| Excitation filter | Semrock | FF02-472/30 | For voltage imaging of super ecliptic pHluorin in Bongwoori |
| Dichroic mirror | Semrock | FF495-Di03-25×36 | |
| Emission filter | Semrock | FF01-497/LP | |
| 75W Xenon arc lamp | CAIRN | OptoSource Illuminator | LEDs and lasers are also effective light sources |
| Slow speed CCD camera | Hitachi | KP-D20BU | |
| Dual port camera adaptor | Olympus | U-DPCAD | |
| High speed CCD camera | RedShirtImaging, LLC | NeuroCCD-SM | |
| Image splitter | CAIRN | Optosplit 2 | |
| Excitation filter | Semrock | FF01-475/23-25 | For voltage imaging of FRET pair based GEVI consisting of Clover and mRuby2) |
| Dichroic mirror | Semrock | FF495-Di03-25×36 | |
| Emission filter | Chroma | ET520/40 | |
| Dichroic mirror | Semrock | FF560-FDi01-25X36 | |
| Emission filter | Chroma | ET645/75 | |
| Vibration isolation system | Kinetic systems | 250BM-IC, 5702E-3036-31 | |
| Patching chamber | Warner instruments | RC-26G, 64-0235 | |
| #0 Micro Coverglass (22x40mm) | Electron Microscopy Sciences | 72198-20 | |
| Temperature controller | Warner instruments | TC-344B | |
| #0 (0.08~0.13mm) – 10mm diameter glass coverslip | Ted Pella | 260366 | |
| Lipofection agent | Life Technologies | 11668-027 | |
| Calcium phosphate reagent | Clontech – Takara | 631312 | |
| Patch clamp amplifier | HEKA | EPC 10 USB amplifier | |
| Multi-channel data acquisition software | HEKA | Patchmaster | |
| Image acquisition and analysis software | RedShirtImaging | Neuroplex | |
| Spreadsheet application software | Microsoft | Microsoft Excel 2010 | |
| Data analysis software | OriginLab | OriginPro 8.6.0 | |
| Demagnifier | Qioptiq LINOS | Optem standard camera coupler 0.38x SC38 J clamp | |
| Confocal microscope | Nikon | Nikon A1R confocal microscope | |
| Anti-fade reagent | Life Technologies | P36930 |
References
- Salzberg, B. M., Davila, H. V., Cohen, L. B. Optical recording of impulses in individual neurones of an invertebrate central nervous system. Nature. 246, 508-509 (1973).
- Cohen, L. B., et al. Changes in axon fluorescence during activity: molecular probes of membrane potential. J. Membrane Biol. 19, 1-36 (1974).
- Tasaki, I., Warashina, A. Dye-membrane interaction and its changes during nerve excitation. Photochem Photobiol. 24, 191-207 (1976).
- Grinvald, A., Hildesheim, R. VSDI: a new era in functional imaging of cortical dynamics. Nat Rev Neurosci. 5, 874-885 (2004).
- Jin, L., Han, Z., Platisa, J., Wooltorton, J. R., Cohen, L. B., Pieribone, V. A. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 75, 779-785 (2012).
- Cao, G., Platisa, J., Pieribone, V. A., Raccuglia, D., Kunst, M., Nitabach, M. N. Genetically targeted optical electrophysiology in intact neural circuits. Cell. 154, 904-913 (2013).
- St-Pierre, F., Marshall, J. D., Yang, Y., Gong, Y., Schnitzer, M. J., Lin, M. Z. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat Neurosci. 17, 884-889 (2014).
- Piao, H. H., Rajakumar, D., Kang, B. E., Kim, E. H., Baker, B. J. Combinatorial mutagenesis of the voltage-sensing domain enables the optical resolution of action potentials firing at 60 Hz by a genetically encoded fluorescent sensor of membrane potential. J Neurosci. 35, 372-385 (2015).
- Jung, A., Garcia, J. E., Kim, E., Yoon, B. J., Baker, B. J. Linker length and fusion site composition improve the optical signal of genetically encoded fluorescent voltage sensors. Neurophoton. 2, 021012 (2015).
- Dimitrov, D., et al. Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS One. 2, e440 (2007).
- Lam, A. J., et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods. 9, 1005-1012 (2012).
- Sung, U., et al. Developing fast fluorescent protein voltage sensors by optimizing FRET interactions. PLoS One. 10, e0141585 (2015).
- Kralj, J. M., Douglass, A. D., Hochbaum, D. R., Maclaurin, D., Cohen, A. E. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat Methods. 9, 90-95 (2012).
- Flytzanis, N. C., et al. Archaerhodopsin variants with enhanced voltage-sensitive fluorescence in mammalian and Caenorhabditis elegans neurons. Nat Commun. 5, 4894 (2014).
- Hochbaum, D. R., et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods. 11, 825-833 (2014).
- Gong, Y., Wagner, M. J., Zhong Li, J., Schnitzer, M. J. Imaging neural spiking in brain tissue using FRET-opsin protein voltage sensors. Nat Commun. 5, 3674 (2014).
- Zou, P., et al. Bright and fast multicoloured voltage reporters via electrochromic FRET. Nat Commun. 5, 4625 (2014).
- Chanda, B., Blunck, R., Faria, L. C., Schweizer, F. E., Mody, I., Bezanilla, F. A hybrid approach to measuring electrical activity in genetically specified neurons. Nat Neurosci. 8, 1619-1626 (2005).
- Wang, D., McMahon, S., Zhang, Z., Jackson, M. B. Hybrid voltage sensor imaging of electrical activity from neurons in hippocampal slices from transgenic mice. J Neurophysiol. 108, 3147-3160 (2012).
- Weigel, S., Flisikowska, T., Schnieke, A., Luksch, H. Hybrid voltage sensor imaging of eGFP-F expressing neurons in chicken midbrain slices. J Neurosci Methods. 233, 28-33 (2014).
- Waters, J. C., Sluder, G., Wolf, D. E. Live-Cell Fluorescence Imaging. Methods in Cell Biology Volume 81. , 115-140 (2007).
- Kaech, S., Banker, G. Culturing hippocampal neurons. Nat Protoc. 1, 2406-2415 (2006).
- Beaudoin, G. M., et al. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc. 7, 1741-1754 (2012).
- Jiang, M., Chen, G. High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat Protoc. 1, 695-700 (2006).
- Molleman, A. . Patch clamping: an introductory guide to patch clamp electrophysiology. , 101-102 (2003).
- Osorio, N., Delmas, P. Patch clamp recording from enteric neurons in situ. Nat Protoc. 6, 15-27 (2010).
- Schroder, M., Kaufman, R. J. The mammalian unfolded protein response. Annu Rev Biochem. 74, 739-789 (2005).
- Wilt, B. A., Fitzgerald, J. E., Schnitzer, M. J. Photon shot noise limits on optical detection of neuronal spikes and estimation of spike timing. Biophys J. 104, 51-62 (2013).
- Lundby, A., Mutoh, H., Dimitrov, D., Akemann, W., Knopfel, T. Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS One. 3, e2514 (2008).
- Peterka, D. S., Takahashi, H., Yuste, R. Imaging voltage in neurons. Neuron. 69, 9-21 (2011).

