- 00:04개요
- 00:52Crystals and X-Ray Diffraction
- 02:14Operation of an X-ray Diffractometer
- 03:25Single Crystal X-ray Diffraction of Mo2(ArNC(H)NAr)4 (where Ar = p-MeOC6H5)
- 04:45Powder X-ray Diffraction
- 05:50Representative Results
- 06:28Applications
- 07:57Summary
单晶和粉末 X 射线衍射
English
소셜에 공유하기
개요
资料来源: 德克萨斯州 #38 大学化学系
x 射线晶体学是一种利用 x 射线来研究分子结构的技术。x 射线衍射 (XRD) 实验通常是用单晶或粉状样品进行的。
单晶 XRD:
单晶 XRD 允许绝对结构的确定。利用单晶 XRD 数据, 可以观察到精确的原子位置, 从而确定键的长度和角度。这种技术提供的结构在一个单一的晶体, 这并不一定代表了大部分的材料。因此, 必须使用额外的体积表征方法来证明化合物的特性和纯度。
粉末 XRD:
与单晶 xrd 不同的是, 粉末 xrd 研究了大量的多晶材料样品, 因此被认为是一种体积表征技术。粉末模式被认为是一种特定材料的 “指纹”;它提供有关材料的相 (变形) 和结晶度的信息。通常, 粉末 XRD 用于研究矿物, 沸石, 金属-有机框架 (MOFs), 和其他扩展固体。粉末 XRD 也可用于建立分子物种的本体纯度。
以前, 我们已经看到如何发展 X 射线质量晶体 (见视频在有机化学精要系列)。在这里我们将学习 XRD 背后的原理。然后, 我们将在 Mo2(ArNC (H)) 的4中收集单晶和粉末数据, 其中 Ar = p-MeOC6H5。
Principles
为什么 X 射线?:
当测量距离时, 重要的是要选择一个度量单位, 它的尺度是被测量的对象。例如, 要测量一支铅笔的长度, 你不会想要使用只有英尺渐变的码棒。同样, 如果一个人想测量一辆汽车的长度, 那么使用一个12英寸的尺和 cm 标记是不合适的。因此, 为了研究分子中的键, 使用与这些键的长度相匹配的光波长是很重要的。X 射线在Å范围内有波长, 与典型的键距 (1-3 Å) 完全匹配。
单元格:
想象一下, 试图描述一支钢笔尖端的所有分子。如果一个近似于它由 6.02 x 1023分子 (或1摩尔) 组成, 那么在分子水平上描述这个物体似乎几乎是不可能的。当一个物体作为晶体存在时, 它的复杂性就被简化了, 其中单元格的内容可以用来描述整个结构。晶体的单元单元是包含一个固体的重复单位的最小体积。它定义为一个长度为 a、b 和 c 的 3D “box”, 以及角度α、β和γ (图 1)。单位细胞允许化学家用一小部分或少量的原子或分子来描述晶体的含量。通过在空间中重复单位单元格, 可以生成实体的3D 表示形式。
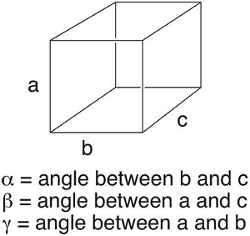
图 1.单元格参数。
实验设置:
单晶和粉末 XRD 有相似的仪器设置。对于单晶 XRD, 晶体是安装和中心在 X 射线束。对于粉末 XRD, 一个多晶样品是研磨成一个细粉和安装在一个板块。样品 (或多晶) 用 x 射线照射, 衍射 x 射线击中探测器。在数据收集过程中, 对 x 射线源和探测器进行旋转。
双缝实验:
回想一下, 光有波浪和粒子般的特性。当单色光进入两个狭缝时, 光的波状性质会在每个狭缝中产生球形的光。当波相互作用时, 它们可以加在一起 (如果波具有相同的波长和相位) 或相互抵消 (如果波具有相同的波长, 但有不同的相位), 这被称为构造和破坏性干扰。由此产生的光的模式是由一系列的线, 其中光区域代表建设性的干扰, 而黑暗地区是破坏性干扰的结果。
典型的衍射模式: 单晶与粉末:
在 X 射线照射晶体时, 辐射在晶体内部与电子密度的相互作用中被衍射。就像经典的缝实验中的水波一样, 衍射的 X 射线相互作用, 产生了建设性的破坏性干扰。在 XRD 中, 衍射图样代表了晶体内原子和键的电子密度。一个典型的单晶衍射模式显示在 (图 2) 中。请注意, 衍射图样是由斑点而不是像双缝实验中的直线组成的。事实上, 这些 “斑点” 是3维球体的2D 片。Crystallographers 使用计算机程序来整合产生的斑点, 以确定衍射 X 射线的形状和强度。在粉末样品中, X 射线在随机方向上与许多微小的晶体相互作用。因此, 观察圆的衍射图样 (图 3), 而不是看到斑点。衍射圆的强度, 然后绘制反对的角度之间的环梁轴 (表示 2θ), 以提供一个2维的情节称为粉末模式。
在这里, 我们将收集单晶和粉末 XRD 数据在 Mo2(ArNC (H) 的),4 , 其中 Ar = p-MeOC6H5, 这是在模块合成的异体 Metal–Metal 的制备和表征保税化合物。
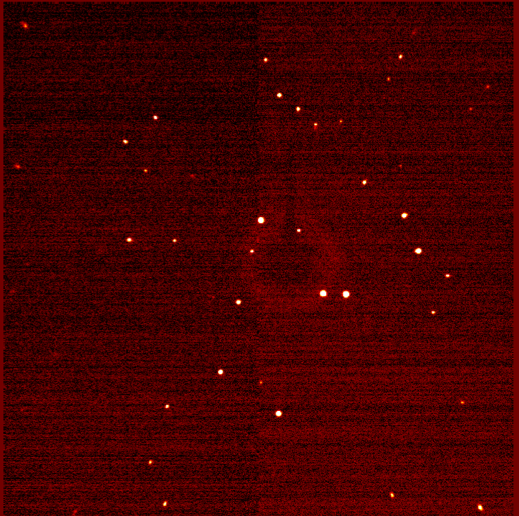
图 2.单晶衍射图样。
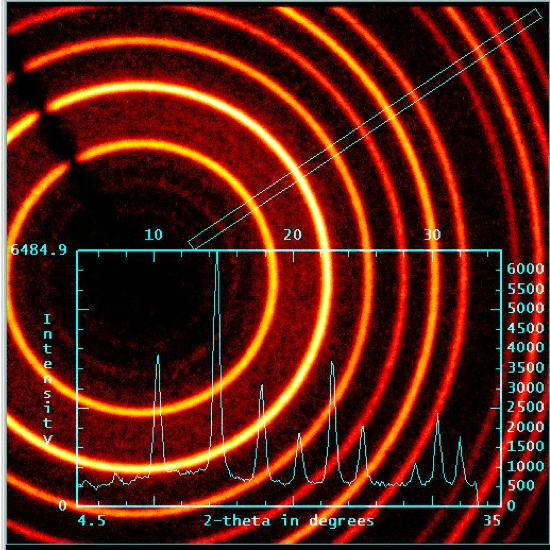
图 3.粉末 XRD: 圆形衍射图样。
Procedure
Results
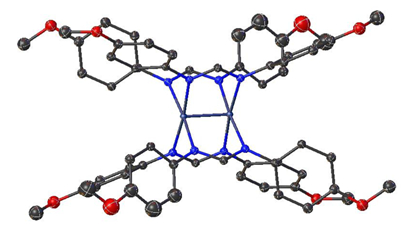
Figure 4. Single-crystal structure of Mo2(ArNC(H)NAr)4 where Ar = p-MeOC6H5.
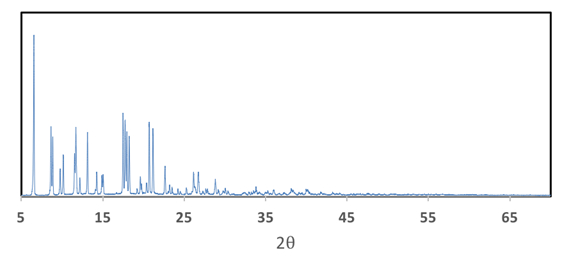
Figure 5. Powder XRD pattern of Mo2(ArNC(H)NAr)4 where Ar = p-MeOC6H5.
Applications and Summary
In this video, we learned about the difference between single-crystal and powder XRD. We collected both single-crystal and powder data on Mo2(ArNC(H)NAr)4, where Ar = p-MeOC6H5.
Single-crystal XRD is a powerful characterization technique that can provide the absolute structure of a molecule. While structure determination is the most common reason chemists use XRD, there are a variety of special X-ray techniques, such as anomalous scattering and photocrystallography, which provide more information about a molecule.
Anomalous scattering can distinguish between atoms of similar molecular weights. This technique is particularly valuable for characterization of heteropolynuclear metal complexes (compounds that have more than one metal atom with different identities). Anomalous scattering has also been used in protein crystallography as a method to help resolve the phase of the diffracted beam, which is important for structure determination.
Photocrystallography involves single-crystal XRD coupled to photochemistry. By irradiating a sample with light in the solid state, we can observe small structural changes and monitor those changes by XRD. Examples of this technique include observing isomerization of a molecule by light as well as characterization of reactive intermediates.
Powder XRD is a non-destructive characterization method that can be used to gain information about the crystallinity of a sample. In addition, it is a useful technique to analyze mixtures of different materials. As previously mentioned, powder patterns are like fingerprints: the resulting pattern of a compound is dependent on how the atoms are arranged within the material. Therefore, an experimentally-determined powder pattern can be compared to a collection of known diffraction patterns of materials in the International Centre for Diffraction Data. This not only provides information about the identity of the product isolated, but also allows scientists to comment on the number of compounds present in the sample. While a majority of the diffraction patterns listed in the database are in the family of extended solids such as minerals and zeolites, examples of inorganic molecules can be found.
내레이션 대본
X-ray diffraction is a common analytical technique used in materials science and biochemistry to determine the structures of crystals.
It traces the paths of X-rays through crystals to probe the structure. There are two major techniques. Powder X-ray diffraction determines the phases and purity of a crystalline species. Single X-ray diffraction identifies the atoms in a crystal and their locations, as well as electron densities, bond lengths, and angles.
This video illustrates the operation of an X-ray diffractometer, procedures for both single-crystal and powder X-ray diffraction, and discusses a few applications.
We’ll start by examining the concept of crystal structure, and exploring how X-rays interact with crystals.
A crystal is a periodic configuration of atoms, that is, a geometric pattern of atoms that repeat at regular intervals. The smallest repeating element of a crystal is called a “unit cell.” It is described by its packing structure, dimensions and bond angles. “Miller indices” describe any fictitious planar cross sections of the unit cell.
X-rays are a form of electromagnetic waves whose wavelengths are similar to the atomic spacing in crystals. When a single X-ray strikes an individual atom, it is diffracted. When two coherent X-rays strike atoms in different planes, the diffracted X-rays interfere, resulting in constructive or destructive signals.
The diffraction pattern of a powder crystalline sample is comprised of intense spots, which form rings of constructive interference. The angles at which these spots occur correspond to the spacing of atoms in that plane. The spacing can be determined using Bragg’s Law.
Now that we have learned about crystals and X-ray diffraction patterns, let’s look at how an X-ray diffractometer works.
An X-ray diffractometer consists of three basic components: an X-ray source, a specimen, and a detector. All components are oriented in a coplanar, circular arrangement with the sample holder at the center. The source usually contains a copper target, that, when bombarded by electrons, emits a beam of collimated X-rays. The beam is directed at the sample, which refracts the X-rays. The sample and the detector are then rotated in opposite directions, until the angles of X-ray intensity are determined.
High X-ray intensity corresponds to constructive interference by a crystallographic plane in both single-crystal and powder X-ray diffraction. Powder X-ray diffraction reveals the crystal structure of the sample, while single-crystal X-ray diffraction additionally reveals the chemical content and locations of atoms.
Now, let us see a practical example of X-ray diffractometry.
Single-crystal X-ray diffraction requires high-quality crystals without impurities, grain boundaries, or other interfacial defects. Bring the crystals of the organo-molybdenum compound to the light microscope to analyze it.
Begin by adding a drop of paratone oil to a clean glass slide. Then add a small amount of paratone oil to a spatula, and scoop some crystals from the crystallization vial onto a slide.
Examine the crystals under the microscope, and select a crystal with uniform, well defined edges. Once an ideal crystal is chosen, use a Kapton loop to pick up the crystal, ensuring little oil sticks to the crystal.
Next, open the diffractometer doors to load the sample. Attach the Kapton loop to the gonoimeter head, centering the crystal with respect to the X-ray beam. Then close the doors.
Open the X-ray crystallography software and run a short data collection sequence that establishes the structure of the unit cell. Based on this data, select a data collection strategy and run full data collection. Once a full data set has been collected, work up the data using a suitable program and refine it.
In comparison to single crystal X-ray diffraction, powder X-ray diffraction is a bulk characterization technique that does not require single crystals.
Choose an appropriately-sized sample holder and a diffraction plate that will not affect the readings at the angles of interest.
Place a fine mesh sieve over the diffraction plate. Carefully add 20 mg of sample to the sieve, keeping the sample over the plate. Tap the sieve on the benchtop until a monolayer of powder forms.
Secure the diffraction plate in the sample holder. Open the diffractometer doors and mount the sample. If the sample mount has locking pins, ensure the pins are engaged and the sample holder is secure before closing the doors.
Using a suitable software, load a standard data collection method. Enter a range of scan angles suitable for the material. Then enter the scan time; a longer scan time allows for better resolution. Then press “start”.
Now, let’s compare the results obtained from the single crystal and powder X-ray diffraction of the organo-molybdenum complex.
From the single crystal X-ray data, a structural model of the electron density map is generated, which is used to obtain experimentally determined bond lengths and angles within the structure.
Furthermore, the powder XRD provides additional information about the compound. The flat baseline of the spectrum indicates, that the sample used is highly crystalline, whereas curved baselines are indicative of amorphous materials.
X-ray diffraction is a valuable characterization tool in virtually every field of material science, and therefore plays a role in diverse applications.
A major component of heritage art conservation includes understanding how works of art were produced and why they corrode. Recent developments in X-ray diffraction study corrosion by destructively testing less than 1 mg of sample. Since corrosion products are rarely monocrystalline, powder X-ray diffraction is required. Typical analyses occur at 2θ between 5-85º degrees over 20 hours. The locations of atoms within the crystal may be optimized algorithmically, providing insight into the location and nature of chemical attacks.
Films of material ranging from nanometers to micrometers in thickness have unique protective, electrical, and optical abilities that differ from those of bulk materials. X-ray diffraction provides information on film thickness, density, and surface texture. It is used to determine film stress, and the likelihood of film failure and breakage. It also helps characterize the optical behavior of films, since absorption largely depends on crystal structure. It is therefore used to characterize thin film light sensors and photovoltaic cells.
You’ve just watched JoVE’s introduction to single-crystal and powder X-ray diffraction. You should now be familiar with the principles of X-ray diffractometry, a procedure for obtaining diffraction patterns, and some applications. As always, thanks for watching!
