通过小鼠肺切除术和假体植入测量内部肺表面积的标准方法
Summary
内肺表面积(ISA)是评估肺部疾病和损伤诱导肺泡再生的肺形态和生理学的关键标准。我们在这里描述了一种标准化方法,可以最大限度地减少肺肺切除术和假体植入小鼠模型中ISA的测量偏差。
Abstract
Pulmonary morphology, physiology, and respiratory functions change in both physiological and pathological conditions. Internal lung surface area (ISA), representing the gas-exchange capacity of the lung, is a critical criterion to assess respiratory function. However, observer bias can significantly influence measured values for lung morphological parameters. The protocol that we describe here minimizes variations during measurements of two morphological parameters used for ISA calculation: internal lung volume (ILV) and mean linear intercept (MLI). Using ISA as a morphometric and functional parameter to determine the outcome of alveolar regeneration in both pneumonectomy (PNX) and prosthesis implantation mouse models, we found that the increased ISA following PNX treatment was significantly blocked by implantation of a prosthesis into the thoracic cavity1. The ability to accurately quantify ISA is not only expected to improve the reliability and reproducibility of lung function studies in injured-induced alveolar regeneration models, but also to promote mechanistic discoveries of multiple pulmonary diseases.
Introduction
肺的基本功能是血管和大气之间的氧气和二氧化碳的交换。肺部疾病,如支气管肺发育不良(BPD),慢性阻塞性肺病(COPD)和急性呼吸道感染,导致降低的ISA 2。研究人员在研究肺病已经开发出多种定量方法来评估肺形态变化,包括美林,ILV,3的气体交换单元的数量,ISA,和肺组织顺应性2。 Weibel 等人的开创性研究4和Duguid 等人 5一起确定了ISA可以作为人肺肺气交换能力的直接指标,可作为确定肺气肿严重程度的标准。过去五年发表的一些研究已经使用肺形态学参数( 例如, </e米> ISA和MLI)对发展6期间和从损伤PNX 1,7恢复期间评估在小鼠的肺中的形态和功能的变化。 ISA使用等式1 8,9计算:
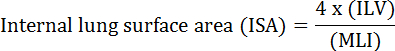
,其中ILV是内部肺容积,MLI是表示肺外周空间大小10的中间参数。
PNX,手术切除的一个或多个肺叶,已被广泛报道,以诱导在许多物种中肺泡再生,包括人类11,小鼠1,狗12,大鼠13,和兔14,15。一个螺柱PNX后14天的小鼠肺显示,既存在肺泡的扩张和肺泡从头形成有助于恢复其余肺组织中的ISA,ILV和肺泡数量1 。我们和其他人已经表明,在PNX( 即 ,假体植入)后,将诸如海绵,蜡或定制假体的材料插入空胸腔会损害肺泡再生。现在已牢固地确立机械力功能的发起肺泡再生1,16,17最重要的因素之一。这些研究强调了使用PNX处理和假体植入肺的ISA值作为定量评估肺泡再生的标准的有效性。
已知观察者偏倚显着影响测量值肺部形态学参数( 例如 ,MIL和ILV)。可以使用标准化协议来消除在确定ILV和MLI中的这种偏差,这是在ISA的计算中使用的两个参数。在这里,我们提供了高度详细的标准化方案来测量这些肺参数。重要的是,准确量化ISA的能力有望提高损伤诱导的肺泡再生模型中肺功能研究的可靠性和可重复性,并且有助于多种肺部疾病的机械发现。
Protocol
Representative Results
Discussion
在本协议中,我们提供关于肺小鼠左肺PNX和假体植入后肺参数测量的详细描述。 ISA现在被认为是评估许多肺部疾病和损伤引起的肺泡再生中呼吸功能的关键指标。然而,虽然肺部研究界对ISA的实用性有一致意见,但迄今为止,几乎没有考虑用于计算ISA的两个参数ILV和MLI的测量标准化。显然,与任何测量一样,重要的是尝试获得无偏见的数据。本研究工作的核心目标是建立一个标准化的方案供小鼠…
Declarações
The authors have nothing to disclose.
Acknowledgements
作者要向北京国家生物科学研究所致谢。这项工作得到了北京市自然科学基金(Z17110200040000)的支持。
Materials
| Low cost cautery kit | Fine Science Tools | 18010-00 | |
| Noyes scissors | Fine Science Tools | 15012-12 | |
| Standard pattern forceps | Fine Science Tools | 11000-12 | |
| Castroviejo Micro Needle Holders | Fine Science Tools | 12060-01 | |
| Vessel clips | Fine Science Tools | 18374-44 | |
| I. V. Cannula-20 gauge | Jinhuan Medical Product Co., LTD. | 29P0601 | |
| Surgical suture | Jinhuan Medical Product Co., LTD. | F602 | |
| Mouse intubation platform | Penn-Century, Inc | Model MIP | |
| Small Animal Laryngoscope | Penn-Century, Inc | Model LS-2-M | |
| TOPO Small Animal Ventilator | Kent Scientific | RSP1006-05L | |
| Thermal pad | Stuart equipment | SBH130D | |
| Pentobarbital sodium salt | Sigma | P3761 | |
| Heparin sodium salt | Sigma | H3393 | |
| Hematoxylin Solution | Sigma | GHS132 | |
| Eosin Y solution, alcoholic | Sigma | HT110116 | |
| 10 ml Pipette | Thermo Scientific | 170356 | |
| Paraformaldehyde | Sigma | P6148 | |
| O.C.T Compound | Tissue-Tek | 4583 | |
| cryosection machine | Leica | CM1950 | |
| Disposable Base Molds | Fisher HealthCare | 22-363-553 | |
| 18 gauge needle | Becton Dickinson | 305199 | |
| Povidone iodine | Fisher Scientific | 19-027132 | |
| 70% ethanol | Fisher Scientific | BP82011 | |
| Infusion sets for single use | Weigao | SFDA 2012 3661704 | |
| Phosphate buffered saline | Gibco | 10010023 | |
| Tapes | 3M Scotch | 8915 | |
| Cotton pad | Vinda | Dr.P | |
| Silicone prosthesis | Custom made | ||
| Brightfield microscope | Olympus | VS120 | |
| Ruler tool | Adobe Photoshop |
Referências
- Liu, Z., et al. MAPK-Mediated YAP Activation Controls Mechanical-Tension-Induced Pulmonary Alveolar Regeneration. Cell Rep. 16 (7), 1810-1819 (2016).
- Thurlbeck, W. M. Internal surface area and other measurements in emphysema. Thorax. 22 (6), 483-496 (1967).
- Knudsen, L., Weibel, E. R., Gundersen, H. J. G., Weinstein, F. V., Ochs, M. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol. 108 (2), 412-421 (2010).
- Weibel, E. R. . Morphometry of the Human Lung. , (1963).
- Duguid, J. B., Young, A., Cauna, D., Lambert, M. W. The internal surface area of the lung in emphysema. J Pathol Bacteriol. 88, 405-421 (1964).
- Branchfield, K., et al. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 351 (6274), 707-710 (2016).
- Ding, B. -. S., et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 147 (3), 539-553 (2011).
- Dunnill, M. S. Quantitative methods in the study of pulmonary pathology. Thorax. 17 (4), 320-328 (1962).
- Weibel, E. R., Gomez, M. Architecture of the human lung. Use of quantitative methods establishes fundamental relations between size and number of lung structures. Science. 137 (3530), 577-585 (1962).
- Thurlbeck, W. M. The internal surface area of nonemphysematous lungs. Am Rev Respir Dis. 95 (5), 765-773 (1967).
- Butler, J. P., et al. Evidence for adult lung growth in humans. N Engl J Med. 367 (16), 244-247 (2012).
- Hsia, C. C. W., Herazo, L. F., Fryder-Doffey, F., Weibel, E. R. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest. 94 (1), 405-412 (1994).
- Thurlbeck, S. W. M. Pneumonectomy in Rats at Various Ages. Am Rev Respir Dis. 120 (5), 1125-1136 (1979).
- Cagle, P. T., Langston, C., Thurlbeck, W. M. The Effect of Age on Postpneumonectomy Growth in Rabbits. Pediatr Pulmonol. 5 (2), 92-95 (1988).
- Langston, C., et al. Alveolar multiplication in the contralateral lung after unilateral pneumonectomy in the rabbit. Am Rev Respir Dis. 115 (1), 7-13 (1977).
- Cohn, R. Factors Affecting The Postnatal Growth of The Lung. Anatomical Record. 75 (2), 195-205 (1939).
- Hsia, C. C., Wu, E. Y., Wagner, E., Weibel, E. R. Preventing mediastinal shift after pneumonectomy impairs regenerative alveolar tissue growth. Am J Physiol Lung Cell Mol Physiol. 281 (5), L1279-L1287 (2001).
- Das, S., MacDonald, K., Chang, H. -. Y. S., Mitzner, W. A simple method of mouse lung intubation. J Vis Exp. (73), e50318 (2013).
- Liu, S., Cimprich, J., Varisco, B. M. Mouse pneumonectomy model of compensatory lung growth. J Vis Exp. (94), (2014).
- Silva, M. F. R., Zin, W. A., Saldiva, P. H. N. Airspace configuration at different transpulmonary pressures in normal and paraquat-induced lung injury in rats. Am J Respir Crit Care Med. 158 (4), 1230-1234 (1998).
- Yilmaz, C., et al. Noninvasive quantification of heterogeneous lung growth following extensive lung resection by high-resolution computed tomography. J Appl Physiol. 107 (5), 1569-1578 (2009).
- Voswinckel, R., et al. Characterisation of post-pneumonectomy lung growth in adult mice. Eur Respir J. 24 (4), 524-532 (2004).
- Ravikumar, P., et al. Regional Lung Growth and Repair Regional lung growth following pneumonectomy assessed by computed tomography. J Appl Physiol. 97, 1567-1574 (2004).
- Gibney, B. C., et al. Detection of murine post-pneumonectomy lung regeneration by 18FDG PET imaging. EJNMMI Res. 2 (1), (2012).
- Muñoz-Barrutia, A., Ceresa, M., Artaechevarria, X., Montuenga, L. M., Ortiz-De-Solorzano, C. Quantification of lung damage in an elastase-induced mouse model of emphysema. Int J Biomed Imaging. 2012, (2012).

