在细胞和组织力学生物学应用一种新型的拉伸平台
Summary
我们在本文中展示了一种新的拉伸平台,可用于研究单个细胞的反应,复杂的各向异性的双轴机械变形和量化生物组织的机械性能。
Abstract
工具,使机械力的应用,细胞和组织,或可量化的生物组织的机械性能有了显着的贡献基本力学生物学的理解。这些技术已被广泛使用,以说明如何各种疾病的发病和进展被很大程度上受到机械线索的影响。本文介绍了一个多功能双轴拉伸(BAXS)平台,可以通过机械刺激单细胞或组织量化的机械刚度。该BAXS平台包括可独立控制的4音圈电机。单细胞可以培养的柔性基板,可以连接到电机允许一个细胞暴露于复杂的,动态的和空间上变化的应变场上。相反,通过将力负载单元,可以同时量化初级组织的机械性能,因为它们暴露于形变周期。在这两种情况下,一组适当的夹具必须设计和安装在BAXS平台马达,以牢牢占据柔性基板或感兴趣的组织。该BAXS平台可以安装在倒置显微镜来执行同时的透射光及/或荧光成像研究中的拉伸实验样品的结构或生物化学反应。本文提供的BAXS平台的设计和使用的实验细节,并提出结果适用于单节和整个组织的研究。该BAXS平台被用来测量细胞核中单个小鼠成肌细胞在响应的变形到基板的应变与测量的离体小鼠主动脉的刚度。该BAXS平台是可以与各种光学显微术以提供在亚细胞,细胞和整体组织水平新颖mechanobiological见解进行组合的多功能工具。
Introduction
机械微环境中许多细胞功能如细胞增殖,迁移和分化,它们在组织的发育和内环境稳定,以及在疾病1-6产生深远的影响中起着重要的作用。多年来,大量的实验工具已被用于机械刺激的细胞或组织,并测量随着我们的基本力学生物学的理解和学习的疾病6-17的发病和进展的目标生物体组织的机械性能。然而,一个人必须经常依赖于几个不同的实验装置,以实现特定的研究的目标。本文介绍了一个单一的,多功能,双轴拉伸(BAXS)平台,使该调查中的作用是机械性能和机械力的亚细胞到整个组织的尺度在发挥生物学研究。该BAXS平台不仅允许quantification个孤立的组织的机械性能,而且也有利于以简单的,复杂的和动态应变场适用于活细胞,以了解他们的反应,拉伸发生在体内的能力。该BAXS平台还维护期间机械测试和扰动对细胞和组织进行活细胞显微镜的能力。
该BAXS平台是可用于研究衬底变形的效果在细胞水平和对生物体组织( 图1A)进 行拉伸试验定制的设备。铝加热器制造,以适应标准的10厘米的培养皿,在37℃下用温度控制器和聚酰亚胺薄膜加热器( 图1B)维护任何生理溶液。这BAXS平台可以集成到一个倒置相差和/或荧光显微镜,并允许同时成像( 图1C)。简言之,BAXS平台包括四个线性音圈马达,其中该可移动部件被安装在沿两个垂直的轴( 图1D)面向微型直线运动球轴承的滑动。线性定位平台被安装到四个马达,以允许将用于( 图1E)的夹紧系统的垂直运动。各电动机的位置是由具有500纳米的分辨率( 图1F)的光学编码器监视。所有的四台电机独立控制,采用光电编码器反馈来执行运动命令( 图1G)运动控制器。 LabVIEW的接口提供了完全控制的位移幅度,速度,以产生细胞或组织样品的完全可定制的,静态的和动态的,变形每个电机的加速。
用于诱导细胞中的形变的方法是通过简单地allowin实现G细胞坚定奉行一个灵活和透明的衬底,然后用四台电机的BAXS平台的拉伸该基板。该BAXS平台允许安装夹附着在基材上的音圈电机的任何自定义设计的一套。为此,我们设计了一套夹具,其灵活和透明基板,制成聚二甲基硅氧烷(PDMS)的,可贴( 图2A-C和图3)。作为夹具将暴露于生理溶液中,所有的份数均用不锈钢机加工,以便灭菌。这些夹具都经过精心设计,以使基底尽可能接近到显微镜物镜以提高图像质量,同时尽量减少应力在基材上伸展( 图2D)时。
同一BAXS平台还可以用于量化的小组织样本的刚度,使用一组合适的夹具与ADAP的特德支持的组织样本和称重传感器监测力量。几种方法可采取安装一个组织到BAXS平台的电机;在这种情况下,不锈钢的昆虫minutiens标签可以为了进行拉伸试验( 图4A-B)通过血管组织中的开口钩。另外,对于粗的组织未经自然开口,组织边缘可以被保持在适当位置与连接到音圈马达或胶合到小玻片用生物胶和附加到与夹具马达夹具。为了进行拉伸测试的微型测力传感器是必需的,并且可以很容易地合并在BAXS平台的电机和用于测量过程中的拉伸循环( 图4C)作用在组织上的力。作为BAXS平台由四个电动机,引入第二负载单元允许一个沿两个正交的方向进行拉伸试验。这种能力允许一个的量化Ý沿两个垂直方向上的相同的实验过程中的单个组织的机械刚度。
重要的是,在所有配置中,将细胞或感兴趣的组织样品始终保持在一个与温度控制槽,都可以访问的用户。这种能力允许样品中引入的药理学试剂的拉伸,以考察样品的时间响应。另外,如在倒置显微镜的光轴保持通畅,各种形式的显微仍可供用户使用。最后,所有的四个马达的BAXS平台为独立是可能的高度可配置的应变场应用到感兴趣的样品。 体内细胞和组织暴露于复杂的和各向异性的拉伸,可以更适当地模仿在这个平台上,而不是传统的单轴拉伸平台7,13,15,18,19。此外,该物理特性的应变场可以动态实验期间改变。这些能力允许用户在检查到一个宽一些的高度复杂的,各向异性的,在时间上和空间上变化的应变场的细胞和组织水平上的反应。本文介绍了BAXS平台的优势和局限性,以及其设计,工作原理和实验细节的单细胞和整个组织的实验。
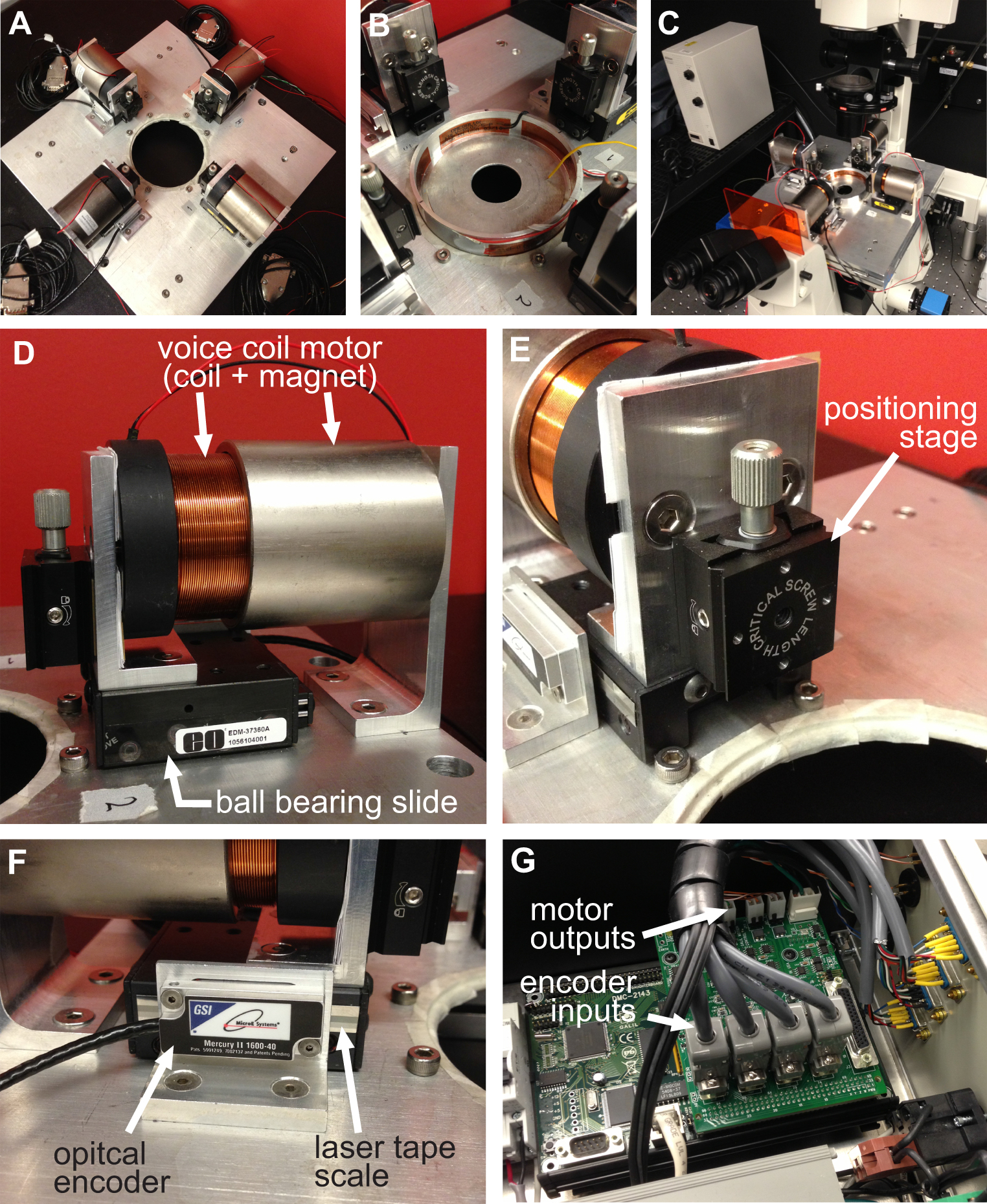
图1概述了BAXS平台。该BAXS平台的 )顶视图显示四个音圈马达用以维持细胞和组织在37°C C)的培养皿加热器该平台可以安装在倒置显微镜进行活的B)详细图片在拉伸实验细胞成像。D)的音圈电机的详细图像;线性定位平台允许夹紧系统的光学编码器提供电机的实时位置,运动控制器。G)的详细图片。F)的详细画面的垂直位移平台的运动部件E)详细图片示出的四个音圈马达的四个光学编码器的输入和功率输出的运动控制器。
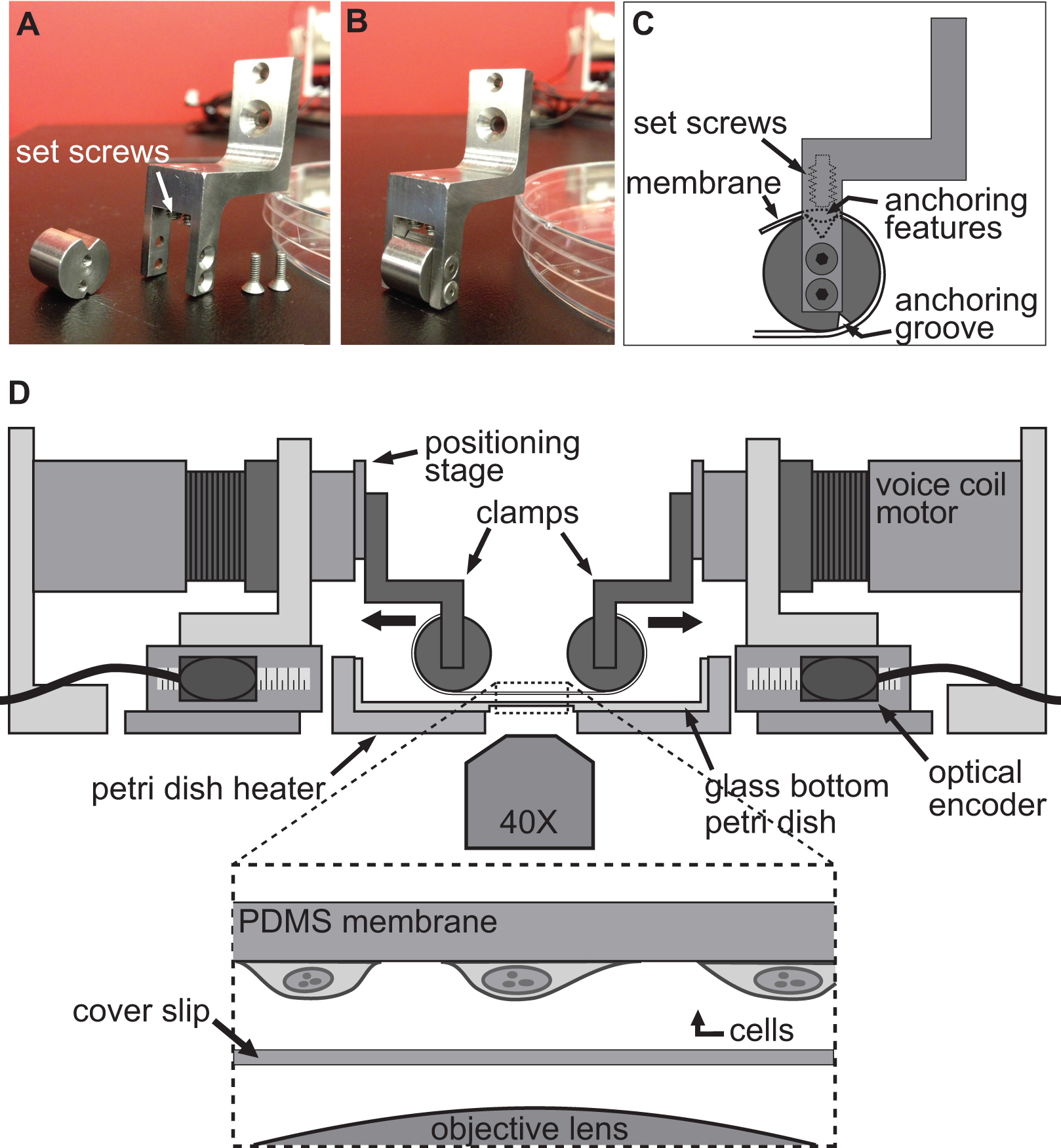
图2。夹紧系统细胞拉伸实验。 A,B)的照片示出用于将PDMS衬底 连接到音圈电机用于拉伸。C)该基片缠绕其锚定的featur夹具的圆筒部的夹具的细节ES坐入槽的顶部。然后将基板使用推基板/锚定功能进入前槽的固定螺丝固定。 四)插图BAXS平台与夹具夹持衬底代替。插图示出了具有连接到它的细胞坐在正上方盖玻片与显微镜物镜的基底的详细视图。
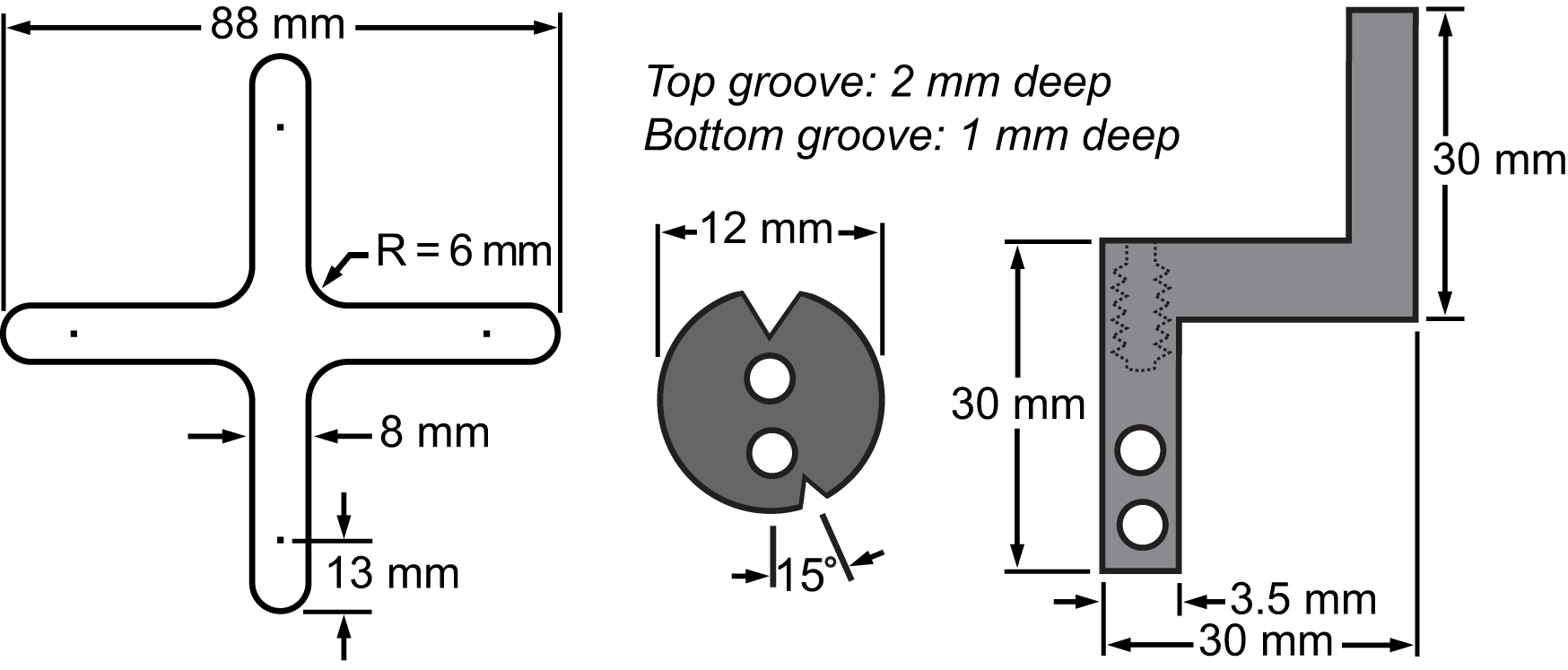
图3的膜和它的夹紧系统的材料清单。图纸表示该主要部分的尺寸集成到双轴平台进行小区拉伸实验。
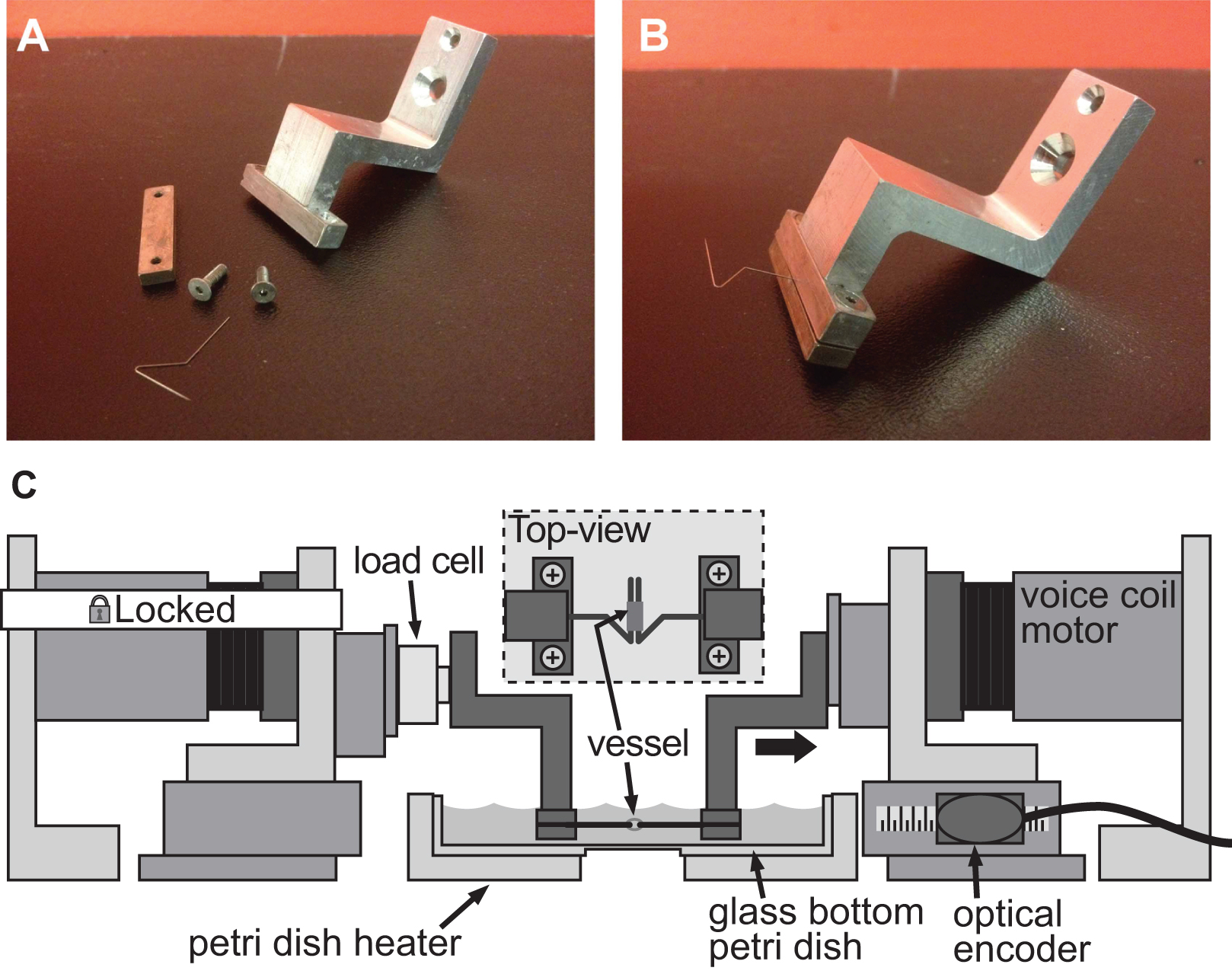
图4实施例充足的夹紧系统,小口径血管僵硬评估用于诱导变形1毫米直径的小鼠主动脉夹紧系统。AB)的详细图片。不锈钢脚都被精心塑造成开放式的三角形,让船只在两个针脚上滑动。C)插图BAXS平台与夹具保持血管和连接固定电机和左钳之间的负载细胞。插图示出了一个详细的顶视图,安装在标签的容器。
Protocol
Representative Results
Discussion
该BAXS平台这里提出有利于在力学生物学的研究中无数次的实验,从单细胞的调查,以整个组织。此外,该平台具有高度的灵活性和可配置性,从而允许多个机械刺激实验和多轴向拉伸试验。该平台还可以使细胞和组织的维持在生理条件下,并在拉伸试验允许同时显微镜。上一节中介绍的两个实验证明BAXS平台的通用性使用一组适当的安装夹时。一种模块化和可定制的样品安装系统,光学连接和添?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
DT是由乐全宗去RECHERCHE魁北克 – 自然等技术(FQRNT)和MITACS升降式战略奖学金博士后奖学金支持。 CMC是由乐全宗去RECHERCHE恩桑特魁北克(FRSQ)和欧内斯特并从渥太华心脏研究所大学玛格丽特福特心脏病学赋研究奖学金博士后奖学金支持。 EOB被授予经营从加拿大卫生研究院(CIHR)MOP80204,并从心T6335和中风安大略基金会的支持。该CIHR及美敦力公司共同提供平等机会条例草案与同行评审的研究主席(URC#57093)。 AEP是由自然科学和工程研究理事会(NSERC)发现格兰特,一个NSERC Discovery Accelerator的补充资金,并衷心感谢加拿大研究主席(CRC)计划和安大略省的早期研究者奖的支持。
Materials
| PDMS | Ellsworth Adhesives | 184 SIL ELAST KIT 0.5KG | The ratio base to cross-linker used in this protocol is 20:1. Mix in a laminar hood to keep dust from contamining your |
| FluoSpheres fluorescent microspheres | Invitrogen | F8810 | Keep away from light. |
| Linear voice coil | Moticont | LVCM-051-051-01 | The motro comes in two pieces (magnet and coil). It has to be mounted on a ball bearing sytem to be functional. |
| Ball bearing slide | Edmund Optics | NT37-360 | Miniature and Small Linear Motion Ball Bearing Slides |
| linear positioning stage | Edmund Optics | 38-960 | Center Drive 1.25" Square Linear Translation Stages |
| optical encoder | GSI microE systems | Mercury II 1600S – 0.5um resolution | reflective incremental encoder. |
| motion controller | Galil | DMC-2143(DIN)-DC48 with AMP-20440 | 4 axis controller with a 4 axis amplifier |
| load cell | Honeywell | 31 low | miniature load cell with a range of 0-150 g |
| Insect minutiens pins (0,20mm) | Pin Service Austerlitz Insect pins | Stainless steel pins that are bended in an opened triangle shape | |
| SU-8 2050 | Micro Chem | SU-8 2050 | Permanent epoxy negative photoresist. Keep away from heat and light |
| Air-plasma treatment system | Glowresearch | Autoglow Oxygen Plasma System | |
| Rat-tail collagen | Invitrogen | A10483-01 | Collagen I, Rat Tail 5mg/ml |
| Hoechst 33342 | Invitrogen | R37605 | DNA-specific fluorescent dye. Keep in the fridge. |
| Kapton (Polyimide Film) Insulated Flexible Heaters | omega.ca | KHLV-0504/(10)-P | 28V flexible heaters; can be supplied with a 24V |
| 1/16 DIN Autotune Temperature and Process Controllers | omega.ca | CN63200-R1-LV | Temperature controller; supply 24 V. |
| DMEM culture medium | Hyclone | SH3024301 | Dulbecco’s Modified 30 Eagle Medium. Keep at 4 C |
| Penicillin-Streptomycin | Hyclone | SV30010 | Keep stock frozen. Keep working solution at 4 C. |
| Fetal bovine serum (FBS) | Hyclone | SH3039603C | Keep frozen. |
| Trypsin 0,05% | Hyclone | SH30236,02 | Keep frozen. Digestion of cell attachement proteins for subcultivation |
| Hepes | Wisent Inc | 330-050-EL | HEPES-buffered salt solution |
| NaCl | Fisher Scientific | BP358-1 | HEPES-buffered salt solution / Krebs physiological solution |
| KCl | Fisher Scientific | BP366-500 | HEPES-buffered salt solution / Krebs physiological solution |
| MgSO4 | Fisher Scientific | M65-500 | HEPES-buffered salt solution / Krebs physiological solution |
| CaCl2 | Fisher Scientific | C614-500 | HEPES-buffered salt solution / Krebs physiological solution |
| Dextrose | Fisher Scientific | BP220-1 | HEPES-buffered salt solution / Krebs physiological solution |
| NaHCO3 | Fisher Scientific | BP328-1 | Krebs physiological solution |
| KH2PO4 | Fisher Scientific | BP362-500 | Krebs physiological solution |
| Carbogen 95% O2/ 5% CO2 | Lindle | DIN:02154749 | Krebs physiological solution oxygenation |
| Nocodazole | Sigma | M1404 | Microtubules depolymerization agent |
| Cytochalasin-D | Sigma | C8273 | Actin filaments depolymerization agent |
| Anti-α-SMA-FITC | Sigma | F3777 | Used to stain and quantify smooth muscle cells content |
| Picrosirius red stain | Fluka | 43665 | Used to stain and quantify collagen content |
References
- Yim, E. K., Sheetz, M. P. Force-dependent cell signaling in stem cell differentiation. Stem Cell Res Ther. 3, (2012).
- Vogel, V., Sheetz, M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 7, 265-275 (2006).
- Wang, N., Tytell, J. D., Ingber, D. E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 10, 75-82 (2009).
- Ingber, D. E. Mechanobiology and diseases of mechanotransduction. Ann Med. 35, 564-577 (2003).
- Janmey, P. A., Miller, R. T. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 124, 9-18 (2011).
- Bukoreshtliev, N. V., Haase, K., Pelling, A. E. Mechanical cues in cellular signalling and communication. Cell Tissue Res. 352, 77-94 (2013).
- Chen, Y., Pasapera, A. M., Koretsky, A. P., Waterman, C. M. Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proc Natl Acad Sci USA. 110, (2013).
- Rosenzweig, D. H., Matmati, M., Khayat, G., Chaudhry, S., Hinz, B., Quinn, T. M. Culture of Primary Bovine Chondrocytes on a Continuously Expanding Surface Inhibits Dedifferentiation. Tissue Eng Part A. 18, 2466-2476 (2012).
- Balachandran, K., et al. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc Natl Acad Sci USA. 108, 19943-19948 (1994).
- Steward, R., Cheng, C. M., Ye, J., Bellin, R., LeDuc, P. Mechanical stretch and shear flow induced reorganization and recruitment of fibronectin in fibroblasts. Sci Rep. 1, (2011).
- Wang, D., Xie, Y., Yuan, B., Xu, J., Gong, P., Jiang, X. A stretching device for imaging real-time molecular dynamics of live cells adhering to elastic membranes on inverted microscopes during the entire process of the stretch. Integr Biol (Camb). 2, 288-293 (2010).
- Haskett, D., Johnson, G., Zhou, A., Utzinger, U., Van de Geest, J. Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomech Model Mechanobiol. 9, 725-736 (2010).
- Duprey, A., Khanafer, K., Schlicht, M., Avril, S., Williams, D., Berguer, R. In vitro characterisation of physiological and maximum elastic modulus of ascending thoracic aortic aneurysms using uniaxial tensile testing. Eur J Vasc Endovasc Surg. 39, 700-707 (2010).
- Tremblay, D., et al. A comparison of mechanical properties of materials used in aortic arch reconstruction. Ann Thorac Surg. 88, 1484-1491 (2009).
- Khanafer, K., Duprey, A., Zainal, M., Schlicht, M., Williams, D., Berguer, R. Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. The Journal of thoracic and cardiovascular surgery. 142, 682-686 (2011).
- Van de Geest, J. P., Sacks, M. S., Vorp, D. A. The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J Biomech. 39, 1324-1334 (2006).
- Guolla, L., Bertrand, M., Haase, K., Pelling, A. E. Force transduction and strain dynamics in actin stress fibres in response to nanonewton forces. J Cell Sci. 125, 603-613 (2012).
- Wang, J. H., Goldschmidt-Clermont, P., Wille, J., Yin, F. C. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech. 34, 1563-1572 (2001).
- Jungbauer, S., Gao, H., Spatz, J. P., Kemkemer, R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J. 95, 3470-3478 (2008).
- Dahl, K. N., Ribeiro, A. J. S., Lammerding, J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 102, 1307-1318 (2008).
- Shivashankar, G. V. Mechanosignaling to the cell nucleus and gene regulation. Annu Rev Biophys. 40, 361-378 (2011).
- Chiquet, M., Gelman, L., Lutz, R., Maier, S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 1793, 911-920 (2009).
- Sullivan, T., et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 147, 913-920 (1999).
- Lammerding, J., et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 113, 370-378 (2004).
- Tremblay, D., Andrzejewski, L., Leclerc, A., Pelling, A. E. Actin and microtubules play distinct roles in governing the anisotropic deformation of cell nuclei in response to substrate strain. Cytoskeleton. , (2013).
- Cuerrier, C. M., et al. Chronic over-expression of heat shock protein 27 attenuates atherogenesis and enhances plaque remodeling: a combined histological and mechanical assessment of aortic lesions. PLoS ONE. 8, (2013).
- Tremblay, D., Cartier, R., Mongrain, R., Leask, R. L. Regional dependency of the vascular smooth muscle cell contribution to the mechanical properties of the pig ascending aortic tissue. J Biomech. 43, 2448-2451 (2010).
- Barker, A. J., Lanning, C., Shandas, R. Quantification of hemodynamic wall shear stress in patients with bicuspid aortic valve using phase-contrast MRI. Ann Biomed Eng. 38, 788-800 (2010).
- Haga, J. H., Li, Y. S. J., Chien, S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 40, 947-960 (2007).
- Frydrychowicz, A., et al. Time-resolved magnetic resonance angiography and flow-sensitive 4-dimensional magnetic resonance imaging at 3 Tesla for blood flow and wall shear stress analysis. The Journal of thoracic and cardiovascular surgery. 136, 400-407 (2008).
- Boccafoschi, F., Mosca, C., Bosetti, M., Cannas, M. The role of mechanical stretching in the activation and localization of adhesion proteins and related intracellular molecules. J Cell Biochem. 112, 1403-1409 (2011).
- Yang, G., Crawford, R. C., Wang, J. H. C. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 37, 1543-1550 (2004).
- Goldyn, A. M., Rioja, B. A., Spatz, J. P., Ballestrem, C., Kemkemer, R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J Cell Sci. 122, 3644-3651 (2009).
- Heo, S. J., et al. Fiber stretch and reorientation modulates mesenchymal stem cell morphology and fibrous gene expression on oriented nanofibrous microenvironments. Ann Biomed Eng. 39, 2780-2790 (2011).
- Zdero, R., et al. Linear and torsional mechanical characteristics of intact and reconstructed scapholunate ligaments. J Biomech Eng. 131, (2009).

