超极化<sup> 13</sup>ç代谢磁共振波谱与成像
Summary
Dynamic nuclear polarization with subsequent sample dissolution has enabled real-time studies of metabolism in biological systems. Hyperpolarized [1-13C]pyruvate was used to study lactate dehydrogenase activity in a prostate carcinoma cell line in vitro.
Abstract
在过去的几十年里,肿瘤分期,再分期,治疗反应的监测,以及多种癌症复发的检测新方法已经出现连同18架F-氟([18 F在国家的最先进的正电子发射断层扫描] -FDG PET)。 13 C核磁共振光谱成像(13 CMRSI)是一种微创成像方法,能够代谢的体内和实时监控。作为与基于13 C核磁共振(NMR)的任何其他方法,它面临低热极化和随后的低信噪比的挑战,因为在13℃和其低天然丰度的相对低的旋磁比生物样品。通过克服这些限制,动态核极化(DNP)与随后的样品溶解最近启用常用NMR和磁共振成像(MRI)系统来测量在各种生物系统的研究中,以及图像关键代谢途径。在13 CMRSI使用一个特别有趣的和有希望的分子是[1- 13 C]丙酮酸盐,其中,在过去的十年里,已被广泛用于体外 ,临床前,以及最近,临床研究调查细胞的能量代谢在癌症和其他疾病。在这篇文章中,我们使用的是3.35牛逼临床DNP超极化勾勒溶解DNP的技术,并展示在体外研究它的用法。类似的协议为超极化可以适用于在体内研究的大部分为好。要做到这一点,我们使用乳酸脱氢酶(LDH)和催化[1- 13 C]丙酮酸使用13 CMRSI [1- 13 C]乳酸盐在前列腺癌细胞系,PC3, 体外代谢反应。
Introduction
目前,为肿瘤分期,再分期,治疗反应监测,复发的检测多种癌症的最广泛使用的临床方法是[18 F] -FDG的PET。 [1]然而,最近,一些新颖的方式和其他方式已经出现。其中的一个方法是13 CMRSI。该技术包括引入13 C-分子成的生物样品,接着微创MRI评估在体外或在实时体内的代谢。尽管如此,13 CMRSI的最大的挑战,相对于其他方法,如[18 F] -FDG的PET或计算机断层扫描,是它的低信噪比。
NMR信号成正比偏振在两个能态的自旋1/2核人口差与总人口的比例( 图1A)的水平。偏振是第一个产品细胞核电子旋磁比(γ)和在温度所施加的磁场强度。的1 H核的典型极化是在0.001%至0.005%的顺序在3 T,它给出了一个相对较差的信噪比。今天的国家的最先进的MRI是一个成功的成像方法,不仅是因为高丰1小时生物样品中和1小时的高旋磁比(γ= 1H兆赫42.576 / T)。然而,观察其他核,如碳,更苛刻。唯一稳定的,磁活性碳同位素,13℃,只占1.1%的所有碳原子。此外,13℃(γ13C = 10.705兆赫/ T)的旋磁比大于1小时的低四倍,从而导致更低的检测效率。总之,低13 C丰度和低γ13C引起热13 C测量来实现的一个1的灵敏度的0.0176%H-NMR测定体内 。
动态核极化
克服13 C测量相对较差的灵敏度的方法是DNP。它是由阿尔伯特·W·欧沃豪斯对金属的最初描述于1953年。在他的文章中,他指出:“这表明,如果传导电子的电子自旋共振是饱和的,细胞核将被极化,以相同的程度,如果他们的旋磁比为使电子自旋的,他们将是”2后来那年,卡弗和Slichter实验证实的Overhauser的假设3。 1958年,Abragam和宝洁描述了这种效应在液体电子和它命名为“坚实的效果。”在温度低于4 K,电子自旋极化达到近100%,是大小比核自旋极化( 图1B)4更高的超过三个数量级。 ŧ发生了由于电子的旋磁比(γE = 28024.944兆赫/ T)为大小比核旋磁比更高的三个数量级。电子和原子核,如Overhauser效应,固体效果,交叉效果,热混合效果之间的弱相互作用,允许极化的传递使用微波照射以频率靠近相应的电子从电子自旋核自旋顺磁共振(EPR)频率5,6。 DNP理论得到了进一步发展涉及到更多的电子和热混合。然而,迄今为止,DNP的不统一的量化理论描述已发表7,8。
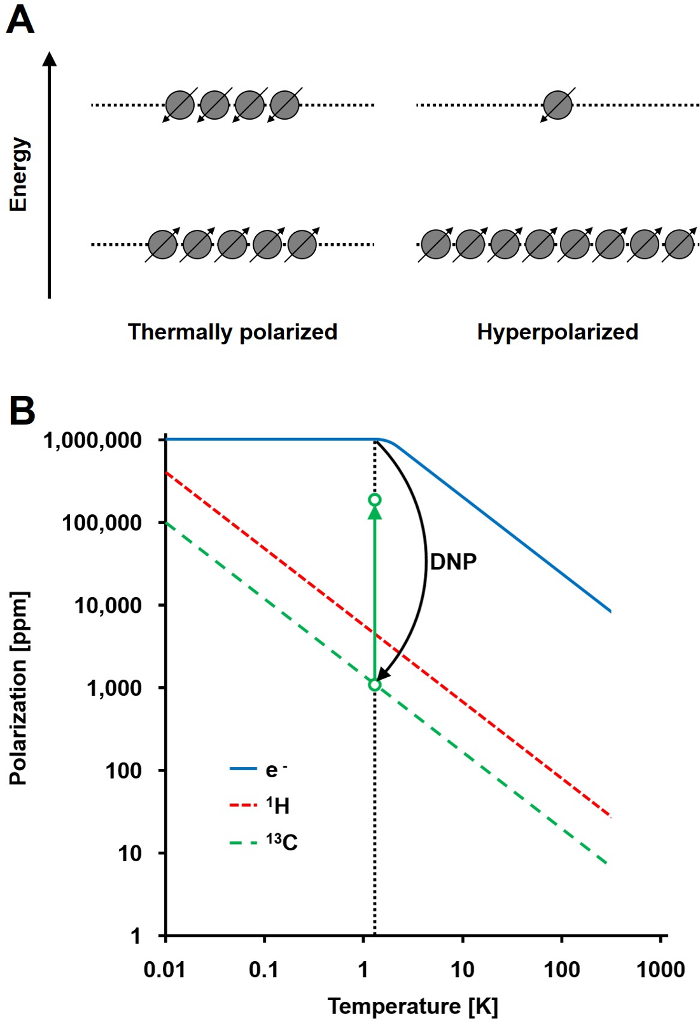
图1:了解动态核极化和超极化。 A)自旋人口的示意图对比在热平衡偏振状态和超极化的状态。 B)中的偏振取决于温度。 (E – )的电子的极化达到低于1.4 K的DNP 100%允许偏振从电子到13 C核,这增加了它们的偏振高达10 5倍的转移。 请点击此处查看该图的放大版本。
在使用13 C-NMR生物系统的研究介绍DNP,随后快速样品溶解已被开发。 50年代以后的Overhauser的假设,扬H. Ardenkjaer拉森等人。解决使超极化冷冻样品到液体状态以最小的超极化损失6的技术上具有挑战性的问题。解散DNP打开一个新的研究领域被称为13 CMRS我,提供了新的方法来研究和描述各种疾病状态9,10。不成对电子,三苯甲基自由基三(8-羧基-2,2,6,6-四(羟基乙基)-benzo- [1,2-4,5] – 双 – (1,3),作为稳定的载体-dithiole -4-基)盐 – 甲基钠(OX063)或(2,2,6,6-四甲基哌啶-1-基)氧基(TEMPO)通常被使用。这些都与期望的13 C标记的分子混合并暴露于微波辐射与接近相应的EPR频率的频率。使用这种技术的13 C核的极化可以增加高达37%11。这导致了10 5倍极化增强相比,热平衡极化11,12。然而,只要在微波辐射停止和/或13 C-分子转移到液体状态,极化与已偏振的13 C核的纵向弛豫时间(T 1)衰减。因此,该快速溶解技术发明或任何后续技术缩短实验测量( 即注射)之前是生物应用13关键的时间。
有迹象表明,候选分子需要满足成功13 CMRSI研究三大要求。第一,所关心的13 C核必须具有足够长的T 1(> 10秒)。的13 C-标签的选择是至关重要的。最佳候选细胞核碳经由键用1 H-细胞核没有直接接触。它也需要在2被迅速代谢- 3个T 1次,导致下游代谢产物与从原始物质的显著不同的化学位移。样品混合物还必须形成无定形固体状态时玻璃,使得空间分布减小电子和13 C之间的距离,从而使反两极分化的外汇储备。如果候选分子不自然地形成非晶化玻璃,它需要在装配玻璃剂高度可溶的,如甘油或二甲基亚砜14。这些要求导致相对小数目的候选分子。然而,即使是合适的分子的成功发现后,开发工作的协议为超极化可以是技术上具有挑战性9,14,15。
近年来,一些衬底已成功偏振光,如[1- 13 C]丙酮酸12,16 – 36,[2- 13 C]丙酮酸37,[1- 13 C]丙酮酸乙酯38,[1- 13 C ]乳酸39,[1- 13 C]富马酸40 – 43,13 C-碳酸氢盐36,44,45,[1- 13 C]乙酸钠43,46 – 49,13 C尿素6,36,50,51 ,[5- 13 C] glutamiNE 15,52,53,[1- 13 C]谷氨酸53,54,[1- 13 C] 2-酮戊二酸55,[1- 13 C]丙氨酸,和其他人14,56。对于超极化一个特别有趣的和常用的基材是[1- 13 C]丙酮酸盐。它被广泛用于临床前研究以调查在各种疾病14,17,22细胞能量代谢。 [1- 13 C]丙酮酸满足所有成功超极化的要求,包括随后被代谢前穿过细胞膜相对长T 1和快速运输。用[1- 13 C]丙酮酸临床前研究,目前正在翻译成诊所57。
丙酮酸代谢
众所周知,有一种癌细胞的DNA,并在其代谢途径变化突变之间的直接联系。早在20世纪20年代,奥托华宝discovERED有葡萄糖和生产乳酸的肿瘤中增加的代谢相比健康组织58 – 60。接着,在其他代谢途径,如戊糖磷酸途径,三羧酸循环,氧化磷酸化,和核苷酸和脂类的合成各种替换,已经描述。
丙酮酸是糖酵解的终产物。在肿瘤,它经历由乳酸脱氢酶61催化的无氧酵解和与辅酶烟酰胺腺嘌呤二核苷酸的还原形式反应 (NADH),产生乳酸和辅酶(NAD +)的氧化形式。可替代地,丙酮酸经过与谷氨酸以形成丙氨酸氨基转移反应,由丙氨酸转氨酶(ALT)的催化。这两种反应是可逆的。丙酮酸也经历脱羧通过丙酮酸脱氢酶(PDH)催化二氧化碳和乙酰-CoA河epresenting在该步骤的不可逆反应。在这些反应速率还可以对与肿瘤代谢17,21,22,25,62。代谢途径总结于图2。

图2:丙酮酸的主要代谢反应的示意图。丙酮酸/乳酸盐转化是通过LDH催化,和丙酮酸/丙氨酸转化是通过ALT催化。丙酮酸不可逆地转化为乙酰-CoA和CO 2通过PDH,和CO 2与碳酸氢盐80 pH依赖平衡。 请点击此处查看该图的放大版本。
超极化[1- 13 C]丙酮酸及其代谢物的检测已经在大鼠先前证实他本领域37,63 – 65,肝脏66,肌肉和肾62,67。一项研究证明了在正常和禁食大鼠肝66之间的乳酸对丙氨酸的比例显著差异,表现在肝癌68,69高度升高和超极化[1- 13 C]乳酸水平。有证据表明,肿瘤分级可以在使用超极化[1- 13 C]丙酮酸22小鼠前列腺(TRAMP)的转基因腺癌被识别,与超极化乳酸水平表示与切除的肿瘤的组织学分级的高相关性。由ALT从丙酮酸催化丙氨酸也被建议作为大鼠肝癌23的有用标志物。
测量丙酮酸乳酸代谢通量已用于监测缺血63,65,70和与细胞毒性化疗17,40,靶向药物对治疗的响应<SUP> 24,25,41,或在动物模型中放疗26。它也被用于检测在成胶质细胞瘤和乳腺癌的小鼠模型25中的磷脂酰肌醇3-激酶(PI3K)抑制剂LY294002反应。在脑丙酮酸代谢的变化肿瘤26和前列腺癌24,71也被治疗后观察到的。
前列腺癌
前列腺癌是男性死亡相关的全球72老年男性和的第二大癌症的主要癌症。迄今为止,还没有可靠的,非侵入性的方法可用于前列腺癌73,74的早期诊断和表征,强调新颖代谢成像技术,迫切需要使严格的检测和患者分期。前列腺癌被用作模型以证明溶解的DNP 13 CMRSI在患者合并的可能性第57条 。这项工作是继续在第一期临床试验采用[1- 13 C]丙酮酸和13 CMRSI为前列腺癌的成像和它最近刚刚已经完成(NCT01229618)。
这背后的工作的动机是更详细和更广泛的受众13 CMRSI方法与细胞的临床前设置应用程序来说明。测定[1- 13 C]丙酮酸LDH催化代谢[1- 13 C]乳酸盐在 PC3前列腺癌细胞系的体外 ,我们表明溶解的DNP在体外研究的可能的应用和处理的关键步骤和在实验过程中的挑战。
Protocol
Representative Results
Discussion
13 CMRSI与超极化探针是实时监测代谢的体外 和体内有前途的方法。其中很重要的一个方面,当使用这种实验过程是适当的标准化,体外实验特别是有关。首先,需要正确地和一致地完成,以实现每个实验超极化材料的相同浓度试样的制备。这需要一个精确称重两种样品是超极化和缓冲。如果浓度是不正确的,该溶液的最终pH是不准确的,它可以有对T 1的细胞的…
Disclosures
The authors have nothing to disclose.
Acknowledgements
E.K. gratefully acknowledges the support of the Graduate School of Bioengineering (GSB) at Technische Universität München. This work was supported by the German Research Foundation (DFG) within the SFB Collaborative Research Center 824, “Imaging for Selection, Monitoring, and Individualization of Cancer Therapies.”
Materials
| HyperSense DNP Polariser | Oxford Instruments | 3.35 T preclinical DNP hyperpolarizer | |
| GE/Agilent MR901 | GE Healthcare/Agilent Technologies | 7 T preclinical MRI scanner, with small bore designed for experiments onrodent | |
| Spinsolve Carbon | Magritek | 1 T NMR spectrometer with permanent magnet | |
| Deuterium Oxide | Sigma Aldrich | 7789-20-0 | |
| Sodium phosphate dibasic | Sigma Aldrich | 7558-79-4 | |
| Sodium phosphate monbasic | Sigma Aldrich | 7558-80-7 | |
| Sodium hydroxide | Sigma Aldrich | 1310-73-2 | |
| Disodium edetate | Sigma Aldrich | 6381-92-6 | |
| Pyruvic acid – 13C1 | Cambridge Isotopes Laboratories | CLM-8077-1 | |
| Dotarem (0.5 mmol/L) | Guerbet | gadoterate meglumine | |
| tris (8-carboxy-2,2,6,6-tetra-(hydroxyethyl)-benzo-[1,2–4,5]-bis-(1,3)-dithiole-4-yl)-methyl sodium salt (OX063) | GE Healthcare | trityl radical used as a sourse of free electron | |
| PC3 cell line | ATCC | CRL1435 | |
| F-12K medium | ATCC | 30-2004 | |
| Fetal Bovine Serum | ATCC | SCRR-30-2020 | |
| Trypsine-EDTA Solution, 1X | ATCC | 30-2101 | |
| Sample plastic cup | Oxford Instruments | ||
| Trypan blue | Bio-Rad | 145-0013-MSDS |
References
- Rohren, E. M., Turkington, T. G., Coleman, R. E. Clinical applications of PET in oncology. Radiology. 231 (2), 305-332 (2004).
- Overhauser, A. W. Polarization of Nuclei in Metals. Phys. Rev. 92 (2), 411-415 (1953).
- Carver, T. R., Slichter, C. P. Polarization of Nuclear Spins in Metals. Phys. Rev. 92 (1), 212-213 (1953).
- Abragam, A., Proctor, W. G. Spin Temperature. Phys. Rev. 109 (5), 1441-1458 (1958).
- Abragam, a., Goldman, M. Principles of dynamic nuclear polarisation. Reports Prog. Phys. 41 (3), 395-467 (2001).
- Ardenkjaer-Larsen, J. H., Fridlund, B., et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 100 (18), 10158-10163 (2003).
- Shimon, D., Hovav, Y., Feintuch, A., Goldfarb, D., Vega, S. Dynamic nuclear polarization in the solid state: a transition between the cross effect and the solid effect. Phys. Chem. Chem. Phys. 14 (16), 5729-5743 (2012).
- Serra, S. C., Rosso, A., Tedoldi, F. Electron and nuclear spin dynamics in the thermal mixing model of dynamic nuclear polarization. Phys. Chem. Chem. Phys. 14 (38), 13299-13308 (2012).
- Gallagher, F. A., Kettunen, M. I., Brindle, K. M. Biomedical applications of hyperpolarized 13C magnetic resonance imaging. Prog. Nucl. Magn. Reson. Spectrosc. 55 (4), 285-295 (2009).
- Hurd, R. E., Yen, Y. -. F., Chen, A., Ardenkjaer-Larsen, J. H. Hyperpolarized 13C metabolic imaging using dissolution dynamic nuclear polarization. J. Magn. Reson. Imaging. 36 (6), 1314-1328 (2012).
- Ardenkjaer-Larsen, J. H., Fridlund, B., et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 100 (18), 10158-10163 (2003).
- Golman, K., in’t Zandt, R., Thaning, M. Real-time metabolic imaging. Proc. Natl. Acad. Sci. 103 (30), 11270-11275 (2006).
- Comment, A., Rentsch, J., et al. Producing over 100 ml of highly concentrated hyperpolarized solution by means of dissolution DNP. J. Magn. Reson. 194 (1), 152-155 (2008).
- Brindle, K. M., Bohndiek, S. E., Gallagher, F. A., Kettunen, M. I. Tumor imaging using hyperpolarized 13C magnetic resonance spectroscopy. Magn. Reson. Med. 66 (2), 505-519 (2011).
- Gallagher, F. A., Kettunen, M. I., Day, S. E., Lerche, M., Brindle, K. M. 13C MR spectroscopy measurements of glutaminase activity in human hepatocellular carcinoma cells using hyperpolarized 13C-labeled glutamine. Magn. Reson. Med. 60 (2), 253-257 (2008).
- Chen, A. P., Albers, M. J., et al. Hyperpolarized C-13 spectroscopic imaging of the TRAMP mouse at 3T-initial experience. Magn. Reson. Med. 58 (6), 1099-1106 (2007).
- Day, S. E., Kettunen, M. I., et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat. Med. 13 (11), 1382-1387 (2007).
- Schroeder, M. A., Swietach, P., et al. Measuring intracellular pH in the heart using hyperpolarized carbon dioxide and bicarbonate: a 13C and 31P magnetic resonance spectroscopy study. Cardiovasc. Res. 86 (1), 82-91 (2010).
- Hurd, R. E., Yen, Y. -. F., Tropp, J., Pfefferbaum, A., Spielman, D. M., Mayer, D. Cerebral dynamics and metabolism of hyperpolarized [1-(13)C]pyruvate using time-resolved MR spectroscopic imaging. J. Cereb. Blood Flow Metab. 30 (13), 1734-1741 (2010).
- Golman, K., Zandt, R. I., Lerche, M., Pehrson, R., Ardenkjaer-Larsen, J. H. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 66 (22), 10855-10860 (2006).
- Park, I., Larson, P. E. Z., et al. Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors. Neuro. Oncol. 12 (2), 133-144 (2010).
- Albers, M. J., Bok, R., et al. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res. 68 (20), 8607-8615 (2008).
- Yen, Y. -. F., Le Roux, P., et al. T(2) relaxation times of (13)C metabolites in a rat hepatocellular carcinoma model measured in vivo using (13)C-MRS of hyperpolarized [1-(13)C]pyruvate. NMR Biomed. 23 (4), 414-423 (2010).
- Dafni, H., Larson, P. E. Z., et al. Hyperpolarized 13C spectroscopic imaging informs on hypoxia-inducible factor-1 and myc activity downstream of platelet-derived growth factor receptor. Cancer Res. 70 (19), 7400-7410 (2010).
- Ward, C. S., Venkatesh, H. S., et al. Noninvasive detection of target modulation following phosphatidylinositol 3-kinase inhibition using hyperpolarized 13C magnetic resonance spectroscopy. Cancer Res. 70 (4), 1296-1305 (2010).
- Day, S. E., Kettunen, M. I., et al. Detecting response of rat C6 glioma tumors to radiotherapy using hyperpolarized [1- 13C]pyruvate and 13C magnetic resonance spectroscopic imaging. Magn. Reson. Med. 65 (2), 557-563 (2011).
- Johannesson, H., Macholl, S., Ardenkjaer-Larsen, J. H. Dynamic Nuclear Polarization of [1-13C]pyruvic acid at 4.6 tesla. J. Magn. Reson. 197 (2), 167-175 (2009).
- Durst, M., Koellisch, U., et al. Bolus tracking for improved metabolic imaging of hyperpolarised compounds. J. Magn. Reson. 243, 40-46 (2014).
- Khegai, O., Schulte, R. F., et al. Apparent rate constant mapping using hyperpolarized [1-(13)C]pyruvate. NMR Biomed. 27 (10), 1256-1265 (2014).
- Sogaard, L. V., Schilling, F., Janich, M. A., Menzel, M. I., Ardenkjaer-Larsen, J. H. In vivo measurement of apparent diffusion coefficients of hyperpolarized (1)(3)C-labeled metabolites. NMR Biomed. 27 (5), 561-569 (2014).
- Aquaro, G. D., Frijia, F., et al. 3D CMR mapping of metabolism by hyperpolarized 13C-pyruvate in ischemia-reperfusion. JACC. Cardiovasc. Imaging. 6 (6), 743-744 (2013).
- Menzel, M. I., Farrell, E. V., et al. Multimodal assessment of in vivo metabolism with hyperpolarized [1-13C]MR spectroscopy and 18F-FDG PET imaging in hepatocellular carcinoma tumor-bearing rats. J. Nucl. Med. 54 (7), 1113-1119 (2013).
- Schilling, F., Duwel, S., et al. Diffusion of hyperpolarized (13) C-metabolites in tumor cell spheroids using real-time NMR spectroscopy. NMR Biomed. 26 (5), 557-568 (2013).
- Schulte, R. F., Sperl, J. I., et al. Saturation-recovery metabolic-exchange rate imaging with hyperpolarized [1-13C] pyruvate using spectral-spatial excitation. Magn. Reson. Med. 69 (5), 1209-1216 (2013).
- Wiesinger, F., Weidl, E., et al. IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C]pyruvate. Magn. Reson. Med. 68 (1), 8-16 (2012).
- Wilson, D. M., Keshari, K. R., et al. Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo. J. Magn. Reson. 205 (1), 141-147 (2010).
- Schroeder, M. A., Atherton, H. J., et al. Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 23 (8), 2529-2538 (2009).
- Hurd, R. E., Yen, Y. -. F., et al. Metabolic imaging in the anesthetized rat brain using hyperpolarized [1-13C] pyruvate and [1-13C] ethyl pyruvate. Magn. Reson. Med. 63 (5), 1137-1143 (2010).
- Chen, A. P., Kurhanewicz, J., et al. Feasibility of using hyperpolarized [1-13C]lactate as a substrate for in vivo metabolic 13C MRSI studies. Magn. Reson. Imaging. 26 (6), 721-726 (2008).
- Witney, T. H., Kettunen, M. I., et al. Detecting treatment response in a model of human breast adenocarcinoma using hyperpolarised [1-(13)C]pyruvate and. Br. J. Cancer. 103 (9), 1400-1406 (2010).
- Bohndiek, S. E., Kettunen, M. I., et al. Detecting tumor response to a vascular disrupting agent using hyperpolarized (13)C magnetic resonance spectroscopy. Mol. Cancer Ther. 9 (12), 3278-3288 (2010).
- Gallagher, F. A., Kettunen, M. I., et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc. Natl. Acad. Sci. U. S. A. 106 (47), 19801-19806 (2009).
- Jensen, P. R., Peitersen, T., et al. Tissue-specific short chain fatty acid metabolism and slow metabolic recovery after ischemia from hyperpolarized NMR in vivo. J. Biol. Chem. 284 (52), 36077-36082 (2009).
- Gallagher, F. A., Kettunen, M. I., et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 453 (7197), 940-943 (2008).
- Scholz, D. J., Janich, M. A., et al. Quantified pH imaging with hyperpolarized 13C-bicarbonate. Magn. Reson. Med. 73 (6), 2274-2282 (2015).
- Koellisch, U., Gringeri, C. V., et al. Metabolic imaging of hyperpolarized [1-(13) C]acetate and [1-(13) C]acetylcarnitine – investigation of the influence of dobutamine induced stress. Magn. Reson. Med. 74 (4), 1011-1018 (2015).
- Koellisch, U., Laustsen, C., et al. Investigation of metabolic changes in STZ-induced diabetic rats with hyperpolarized [1-13C]acetate. Physiol. Rep. 3 (8), (2015).
- Jensen, P. R., Meier, S., Ardenkjaer-Larsen, J. H., Duus, J. O., Karlsson, M., Lerche, M. H. Detection of low-populated reaction intermediates with hyperpolarized NMR. Chem. Commun. (34), 5168-5170 (2009).
- Koelsch, B. L., Keshari, K. R., Peeters, T. H., Larson, P. E. Z., Wilson, D. M., Kurhanewicz, J. Diffusion MR of hyperpolarized 13C molecules in solution. Analyst. 138 (4), 1011-1014 (2013).
- Golman, K., Ardenkjaer-Larsen, J. H., Petersson, J. S., Mansson, S., Leunbach, I. Molecular imaging with endogenous substances. Proc. Natl. Acad. Sci. U. S. A. 100 (18), 10435-10439 (2003).
- von Morze, C., Larson, P. E. Z., et al. Imaging of Blood Flow Using Hyperpolarized [(13)C]Urea in Preclinical Cancer Models. J. Magn. Reson. Imaging. 33 (3), 692-697 (2011).
- Chiavazza, E., Kubala, E., et al. Earth’s magnetic field enabled scalar coupling relaxation of 13C nuclei bound to fast-relaxing quadrupolar 14N in amide groups. J. Magn. Reson. 227, 35-38 (2013).
- Jensen, P. R., Karlsson, M., Meier, S., Duus, J., Lerche, M. H. Hyperpolarized amino acids for in vivo assays of transaminase activity. Chem. – A Eur. J. 15 (39), 10010-10012 (2009).
- Gallagher, F. A., Kettunen, M. I., et al. Detection of tumor glutamate metabolism in vivo using 13C magnetic resonance spectroscopy and hyperpolarized [1-13C]glutamate. Magn. Reson. Med. 66 (1), 18-23 (2011).
- Chaumeil, M. M., Larson, P. E. Z., et al. Hyperpolarized [1-13C] glutamate: a metabolic imaging biomarker of IDH1 mutational status in glioma. Cancer Res. 74 (16), 4247-4257 (2014).
- Keshari, K. R., Wilson, D. M. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 43 (5), 1627-1659 (2014).
- Nelson, S. J., Kurhanewicz, J., et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 5 (198), (2013).
- Warburg, O. On the origin of cancer cells. Science. 123 (3191), 309-314 (1956).
- Warburg, O., Wind, F., Negelein, E. {Ü}ber den Stoffwechsel von Tumoren im K{ö}rper. Klin. Wochenschr. 5 (19), 829-832 (1926).
- Barnes, A. B., De Paepe, G., et al. High-Field Dynamic Nuclear Polarization for Solid and Solution. Biological NMR. Appl. Magn. Reson. 34 (3-4), 237-263 (2008).
- Koukourakis, M. I., Giatromanolaki, A., Sivridis, E., Gatter, K. C., Harris, A. L. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia. 7 (1), 1-6 (2005).
- Golman, K., Petersson, J. S. Metabolic Imaging and Other Applications of Hyperpolarized 13C1. Acad. Radiol. 13 (8), 932-942 (2016).
- Golman, K., Petersson, J. S., et al. Cardiac metabolism measured noninvasively by hyperpolarized 13C MRI. Magn. Reson. Med. 59 (5), 1005-1013 (2008).
- Merritt, M. E., Harrison, C., Storey, C., Jeffrey, F. M., Sherry, A. D., Malloy, C. R. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc. Natl. Acad. Sci. U. S. A. 104 (50), 19773-19777 (2007).
- Merritt, M. E., Harrison, C., Storey, C., Sherry, A. D., Malloy, C. R. Inhibition of carbohydrate oxidation during the first minute of reperfusion after brief ischemia NMR detection of hyperpolarized 13CO2 and H13CO3-. Magn. Reson. Med. 60 (5), 1029-1036 (2008).
- Hu, S., Chen, A. P., et al. In vivo carbon-13 dynamic MRS and MRSI of normal and fasted rat liver with hyperpolarized 13C-pyruvate. Mol. imaging Biol. MIB Off. Publ. Acad. Mol. Imaging. 11 (6), 399-407 (2009).
- Kohler, S. J., Yen, Y., et al. In vivo 13 carbon metabolic imaging at 3T with hyperpolarized 13C-1-pyruvate. Magn. Reson. Med. 58 (1), 65-69 (2007).
- Hu, S., Lustig, M., et al. 3D compressed sensing for highly accelerated hyperpolarized (13)C MRSI with in vivo applications to transgenic mouse models of cancer. Magn. Reson. Med. 63 (2), 312-321 (2010).
- Kurhanewicz, J., Vigneron, D. B., et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia. 13 (2), 81-97 (2011).
- Schroeder, M. A., Cochlin, L. E., Heather, L. C., Clarke, K., Radda, G. K., Tyler, D. J. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc. Natl. Acad. Sci. U. S. A. 105 (33), 12051-12056 (2008).
- Zierhut, M. L., Yen, Y. -. F., et al. Kinetic modeling of hyperpolarized 13C1-pyruvate metabolism in normal rats and TRAMP mice. J. Magn. Reson. 202 (1), 85-92 (2010).
- Dennis, L. K., Resnick, M. I. Analysis of recent trends in prostate cancer incidence and mortality. Prostate. 42 (4), 247-252 (2000).
- Jambor, I., Borra, R., et al. Functional imaging of localized prostate cancer aggressiveness using 11C-acetate PET/CT and 1H-MR spectroscopy. J. Nucl. Med. 51 (11), 1676-1683 (2010).
- Presti, J. C. J., Hricak, H., Narayan, P. A., Shinohara, K., White, S., Carroll, P. R. Local staging of prostatic carcinoma: comparison of transrectal sonography and endorectal MR imaging. AJR. Am. J. Roentgenol. 166 (1), 103-108 (1996).
- Schulte, R. F., Sacolick, L., et al. Transmit gain calibration for nonproton MR using the Bloch-Siegert shift. NMR Biomed. 24 (9), 1068-1072 (2011).
- Durst, M., Koellisch, U., et al. Comparison of acquisition schemes for hyperpolarised (1)(3)C imaging. NMR Biomed. 28 (6), 715-725 (2015).
- Janich, M. A., Menzel, M. I., et al. Effects of pyruvate dose on in vivo metabolism and quantification of hyperpolarized (1)(3)C spectra. NMR Biomed. 25 (1), 142-151 (2012).
- Harrison, C., Yang, C., et al. Comparison of kinetic models for analysis of pyruvate-to-lactate exchange by hyperpolarized 13 C NMR. NMR Biomed. 25 (11), 1286-1294 (2012).
- Gómez Damián, P. A., Sperl, J. I., et al. Multisite Kinetic Modeling of (13)C Metabolic MR Using [1-(13)C]Pyruvate. Radiol. Res. Pract. 2014, (2014).
- Talbot, J. -. N., Gutman, F., et al. PET/CT in patients with hepatocellular carcinoma using [(18)F]fluorocholine: preliminary comparison with [(18)F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 33 (11), 1285-1289 (2006).
- Reeder, S. B., Pineda, A. R., et al. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn. Reson. Med. 54 (3), 636-644 (2005).
- Laustsen, C., Ostergaard, J. A., et al. Assessment of early diabetic renal changes with hyperpolarized [1-(13) C]pyruvate. Diabetes. Metab. Res. Rev. 29 (2), 125-129 (2013).
- Serrao, E. M., Brindle, K. M. Potential Clinical Roles for Metabolic Imaging with Hyperpolarized [1-(13)C]Pyruvate. Front. Oncol. 6, 59 (2016).

