Atomic Force Microscopy to Study the Physical Properties of Epidermal Cells of Live Arabidopsis Roots
Summary
The atomic force microscopy indentation protocol offers the possibility to dissect the role of the physical properties of the cell wall of a particular cell of a tissue or organ during normal or constrained growth (i.e., under water deficit).
Abstract
A method is described here to characterize the physical properties of the cell wall of epidermal cells of living Arabidopsis roots through nanoindentations with an atomic force microscope (AFM) coupled with an optical inverted fluorescence microscope. The method consists of applying controlled forces to the sample while measuring its deformation, allowing quantifying parameters such as the apparent Young’s modulus of cell walls at subcellular resolutions. It requires a careful mechanical immobilization of the sample and correct selection of indenters and indentation depths. Although it can be used only in external tissues, this method allows characterizing mechanical changes in plant cell walls during development and enables the correlation of these microscopic changes with the growth of an entire organ.
Introduction
Plant cells are surrounded by a cell wall that is a complex structure composed of interacting networks of polysaccharides, proteins, metabolites, and water that varies in thickness from 0.1 to several µm depending on the cell type and the phase of growth1,2. Cell wall mechanical properties play an essential role in the growth of plants. Low stiffness values of the cell wall have been proposed as a precondition for cell growth and cell-wall expansion, and there is increasing evidence that all cells sense mechanical forces to perform their functions. However, it is still debated whether changes in the physical properties of the cell wall determines cell fate2,3,4. Because plant cells do not move during development, the final shape of an organ depends on how far and in what direction a cell expands. Thus, Arabidopsis root is a good model to study the impact of cell wall physical properties in cell expansion because different types of expansion occur in different regions of the root. For example, anisotropic expansion is evident in the elongation zone and particularly noticeably in the epidermal cells5.
The method described here was used to characterize the physical properties of the cell wall of epidermal cells at the nanoscale of living Arabidopsis roots using an Atomic Force Microscope (AFM) coupled with an inverted fluorescence phase microscope6. For an extensive revision of the AFM technique, read7,8,9.
This protocol outlines a basic sample preparation method and a general method for AFM-based elasticity measurements of plant cell walls.
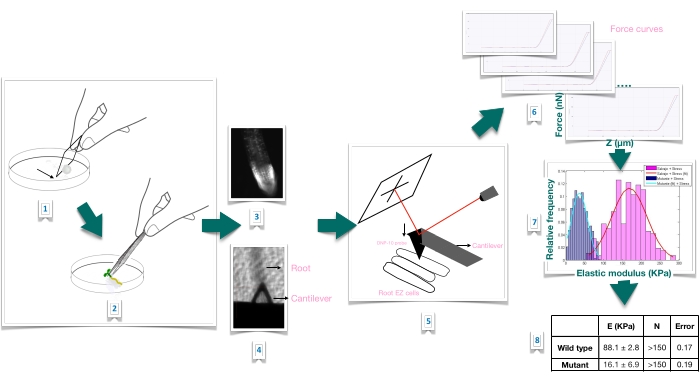
Figure 1: Schematic overview of force-indentation experiment in Arabidopsis roots using atomic force microscopy (AFM). The scheme gives an overview of the steps of a Force-Indentation experiment from the preparation of the substrate to immobilize the root sample firmly (1-2), root viability confirmation through propidium iodide staining (3), cantilever positioning on the surface of an elongated epidermal cell of the primary root (4-5), force curves measurement (6), and force curve processing to calculate the apparent Young's modulus (7-8). EZ: elongation zone. Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
Cell and cell-wall mechanics are increasingly becoming relevant to gain insight into how mechanics affects growth processes. As physical forces propagate over considerable distances in solid tissues, the study of changes in the physical properties of the cell wall and how they are sensed, controlled, tuned, and impact the plant's growth are becoming an important field of study2,3,8.
A method is pr…
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was funded by CSIC I+D 2018, grant No. 95 (Mariana Sotelo Silveira).; CSIC Grupos (Omar Borsani) and PEDECIBA.
Materials
| 1 x Phosphate-Buffered Saline (PBS) | Include sodium chloride and phosphate buffer and is formulated to prevent osmotic shock and maintain water balance in living cells. | ||
| AFM software | Bruker, Billerica, MA, USA | ||
| Atomic force microscopy (AFM) | BioScope Catalyst, Bruker, Billerica, MA, USA | ||
| Catalyst Probe holder-fluid | Bruker, Billerica, MA, USA | CAT-FCH | A probe holder for the Bioscope Catalyst, designed for fluid operation in contact or Tapping Mode. Also compatible with air operation. |
| Cryoscopic osmometer; model OSMOMAT 030 | Gonotech, Berlin, Germany | ||
| Murashige & Skoog Medium | Duchess Biochemie | M0221 | Original concentration, (1962) |
| Optical inverted microscope coupled to the AFM | Olympus IX81, Miami, FL, USA | ||
| PEGAMIL | ANAEROBICOS S.R.L., Buenos Aires, Argentina | 100429 | Neutral, non acidic silicone glue |
| Petri dishes | Deltalab | 200201.B | Polystyrene, 55 x 14 mm, radiation sterile. |
| Propidium iodide | Sigma | P4170 | For root viability test. |
| Silicon nitride probe, DNP-10, cantilever A | Bruker, Billerica, MA, USA | DNP-10/A | For force modulation microscopy in liquid operation. Probe tip radius of 20-60 nm. 175-μm-long triangular cantilever, with a spring constant of 0.35 N/m. |
| Tweezers | Sigma | T4537 |
References
- Anderson, C. T., Kieber, J. J. Dynamic construction, perception, and remodeling of plant cell walls. Annual Review of Plant Biology. 71, 39-69 (2020).
- Roeder, A. H. K., et al. Fifteen compelling open questions in plant cell biology. The Plant Cell. 34 (1), 72-102 (2022).
- Zhang, B., Gao, Y., Zhang, L., Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. Journal of Integrative Plant Biology. 63 (1), 251-272 (2021).
- Hamant, O., Haswell, E. S. Life behind the wall: Sensing mechanical cues in plants. BMC Biology. 15 (59), 1-9 (2017).
- Scheres, B., Benfey, P., Dolan, L. Root development. The Arabidopsis Book. 1, 0101 (2002).
- Cuadrado-Pedetti, M. B., et al. The arabidopsis tetratricopeptide thioredoxin-like 1 gene is involved in anisotropic root growth during osmotic stress adaptation. Genes. 12 (2), 236 (2021).
- Milani, P., Braybrook, S. A., Boudaoud, A. Shrinking the hammer: micromechanical approaches to morphogenesis. Journal of Experimental Botany. 64 (15), 4651-4662 (2013).
- Braybrook, S. A. Measuring the elasticity of plant cells with atomic force microscopy. Methods in Cell Biology. 125, 237-254 (2015).
- Bidhendi, A. J., Geitmann, A. Methods to quantify primary plant cell wall mechanics. Journal of Experimental Botany. 70 (14), 3615-3648 (2019).
- Desnos, T., et al. Procuste1 mutants identify two distinct genetic pathways controlling hypocotyl cell elongation, respectively in dark- and light-grown Arabidopsis seedlings. Development. 122 (2), 683-693 (1996).
- Fagard, M., et al. Procuste1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of arabidopsis. The Plant Cell. 12 (12), 2409-2423 (2000).
- Murashige, T., Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 15 (3), 473-497 (1962).
- Perilli, S., Sabatini, S. Analysis of root meristem size development. Methods in Molecular Biology. 655, 177-187 (2010).
- Sader, J. E., et al. A virtual instrument to standardise the calibration of atomic force microscope cantilevers. Review of Scientific Instruments. 87 (9), 093711 (2016).
- Collinsworth, A. M., Zhang, S., Kraus, W. E., Truskey, G. A. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. American Journal of Physiology – Cell Physiology. 283 (4), 1219-1227 (2002).
- Mathur, A. B., Collinsworth, A. M., Reichert, W. M., Kraus, W. E., Truskey, G. A. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. Journal of Biomechanics. 34 (12), 1545-1553 (2001).
- Sirghi, L., Ponti, J., Broggi, F., Rossi, F. Probing elasticity and adhesion of live cells by atomic force microscopy indentation. European Biophysics Journal. 37 (6), 935-945 (2008).
- Peaucelle, A. AFM-based mapping of the elastic properties of cell walls: At tissue, cellular, and subcellular resolutions. Journal of Visualized Experiments: JoVE. (89), e51317 (2014).
- Peaucelle, A., et al. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Current Biology. 21 (20), 1720-1726 (2011).
- Fernandes, A. N., et al. Mechanical properties of epidermal cells of whole living roots of Arabidopsis thaliana: An atomic force microscopy study. Physical Review E. 85 (2), 21916 (2012).

