在纳米和微米大小的囊泡中构建失衡代谢网络
Summary
我们提出了一种用于在亚微米和微米大小的脂质囊泡中重建膜蛋白并将酶和其他水溶性成分封装的方案。
Abstract
我们提出了一种将复杂的蛋白质网络掺入囊泡的方法,该网络涉及整合的膜蛋白、酶和基于荧光的传感器,使用纯化的组分。该方法对于生物反应器的设计和构建以及复杂失衡代谢反应网络的研究具有重要意义。我们首先根据先前开发的方案将(多个)膜蛋白重构为大的单层囊泡 (LUV)。然后,我们通过冻融挤出封装纯化的酶、代谢物和基于荧光的传感器(荧光蛋白或染料)的混合物,并通过离心和/或尺寸排阻色谱法去除未掺入的组分。通过监测 ATP/ADP 比率、代谢物浓度、内部 pH 值或其他荧光读数参数,实时测量代谢网络的性能。我们直径为 100-400 nm 的含膜蛋白囊泡可以使用现有但优化的程序转化为巨单层囊泡 (GUV)。该方法能够将可溶性成分(酶、代谢物、传感器)包含在微米大小的囊泡中,从而将生物反应器的体积增加几个数量级。含有GUV的代谢网络被捕获在微流体装置中,通过光学显微镜进行分析。
Introduction
自下而上的合成生物学领域侧重于构建(最小)细胞 1,2 和用于生物技术 3,4 或生物医学目的的代谢生物反应器 5,6,7,8。合成细胞的构建提供了一个独特的平台,使研究人员能够在模仿天然环境的明确条件下研究(膜)蛋白质,从而能够发现蛋白质和反应网络的涌现特性和隐藏的生化功能9。作为迈向自主功能合成细胞的中间步骤,开发的模块可以捕获活细胞的基本特征,例如代谢能量守恒、蛋白质和脂质合成以及体内平衡。这些模块不仅增强了我们对生命的理解,而且在医学8和生物技术10领域也有潜在的应用。
跨膜蛋白几乎是任何代谢网络的核心,因为它们将分子运入或运出细胞、发出信号并对环境质量做出反应,并发挥许多生物合成作用。因此,在大多数情况下,合成细胞中代谢模块的工程需要将整合和/或外周膜蛋白重组为由特定脂质和高完整性(低渗透性)组成的膜双层。这些膜蛋白的处理具有挑战性,需要特定的知识和实验技能。
已经开发了几种方法来重建磷脂囊泡内的膜蛋白,最常见的目的是研究特定蛋白质的功能11,12、调节13、动力学特性14,15、脂质依赖性15,16 和/或稳定性17。这些方法包括在脂质存在下将洗涤剂溶解的蛋白质快速稀释到水性介质中18,通过将洗涤剂溶解的蛋白质与洗涤剂不稳定的脂质囊泡孵育来去除洗涤剂,并将洗涤剂吸收到聚苯乙烯珠19 上,或通过透析或尺寸排阻色谱法 20 去除洗涤剂20.有机溶剂已被用于形成脂质囊泡,例如,通过形成油水界面相21,但是当暴露于此类溶剂时,大多数整合膜蛋白会失活。
在我们的实验室中,我们主要通过洗涤剂吸收法重组膜蛋白以形成大单层囊泡 (LUV)19。该方法允许多种膜蛋白的共重组以及酶、代谢物和探针在囊泡腔中的包封22,23。含有膜蛋白的 LUV 可以在有/不包封水溶性成分的情况下转化为巨单层囊泡 (GUV),使用电形成24 或凝胶辅助溶胀25 和特定条件来保持膜蛋白的完整性26。
本文提出了一种在LUV中重建失衡代谢网络的方案,该网络通过将L-精氨酸分解成L-鸟氨酸27来再生ATP。ATP 的形成与甘油-3-磷酸 (G3P) 的产生偶联,甘油-3-磷酸是磷脂合成的重要组成部分22,28。代谢途径由两种完整的膜蛋白组成,即精氨酸/鸟氨酸 (ArcD) 和 G3P/Pi 逆向转运蛋白 (GlpT)。此外,ATP的循环需要三种可溶性酶(ArcA、ArcB、ArcC),GlpK用于将甘油转化为3-磷酸甘油,利用来自L-精氨酸分解的ATP,见图1了解该途径的示意图。该协议为未来构建更复杂的反应网络(用于脂质或蛋白质的合成或细胞分裂)提供了一个良好的起点。囊泡的脂质组成支持多种整合膜蛋白的活性,并且已针对不同分子进出囊泡的转运进行了优化 27,29,30。
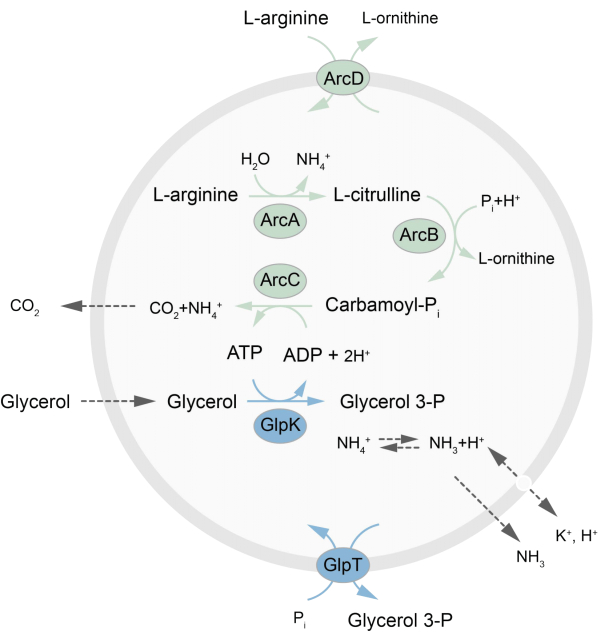
图 1:ATP 产生和甘油 3-磷酸合成和排泄的途径概述。 请点击这里查看此图的较大版本.
简而言之,将纯化的膜蛋白(溶解在十二烷基-β-D-麦芽糖苷,DDM)中加入到已用Triton X-100不稳定的预形成的脂质囊泡中,该脂质囊泡允许将蛋白质插入膜中。洗涤剂分子随后通过添加活化的聚苯乙烯珠子(缓慢)去除,从而形成密封良好的蛋白脂质体。然后可以将可溶性成分添加到囊泡中,并通过冻融循环进行封装,从而在膜融合过程中捕获分子。获得的囊泡是高度异质的,许多是多层状的。然后,它们通过孔径为 400、200 或 100 nm 的聚碳酸酯过滤器挤出,从而产生尺寸更均匀的囊泡;孔径越小,囊泡越均匀和单层状,但代价是内部体积较小。通过体积排阻色谱法从外部溶液中去除未掺入的蛋白质和小分子。proteoLUVs可以通过凝胶辅助溶胀转化为微米大小的囊泡,然后这些proteoGUVs被收集并捕获在微流控芯片中,用于显微表征和操作。 图 2 显示了完整协议的示意图概述。
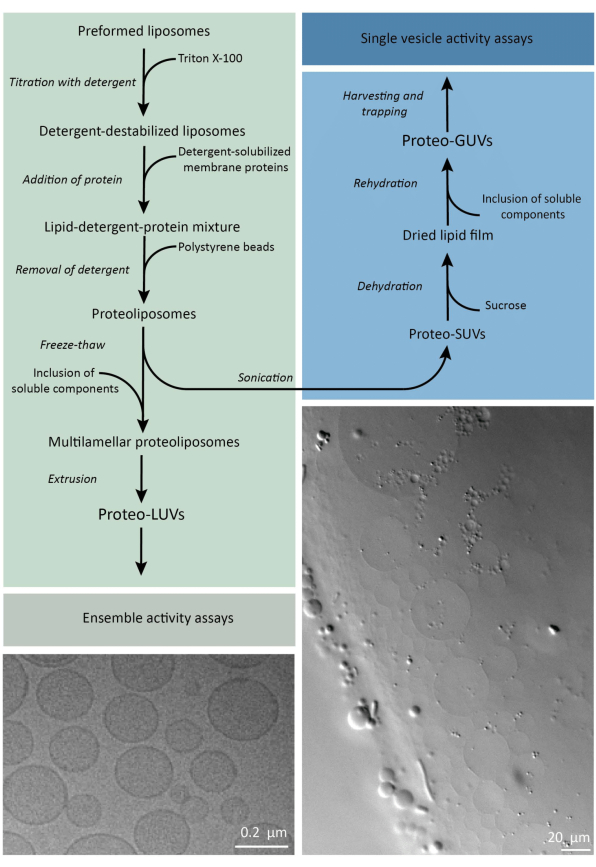
图 2:在亚微米 (LUV) 和微米尺寸 (GUV) 的脂质囊泡中重建膜蛋白和封装酶和水溶性成分的方案概述。 请点击这里查看此图的较大版本.
重组和包封方案效果良好,保留了蛋白质的功能,但 proteoLUV 和 proteoGUV 的大小是异质的。微流体方法31,32 允许形成微米大小的囊泡,这些囊泡的大小更均匀,但膜蛋白的功能重建通常是不可能的,因为双层中的残留溶剂会使蛋白质失活。proteoLUV 的大小范围为 100 至 400 nm,在低浓度的酶下,包封可能导致具有不完整代谢途径的囊泡(随机效应;见图 3)。LUV 是构建特定代谢模块的理想选择,如图所示,用于生产 ATP 和 G3P 等构建块。这种蛋白 LUV 有可能被包裹在 GUV 中,并作为宿主囊泡的细胞器样隔室。
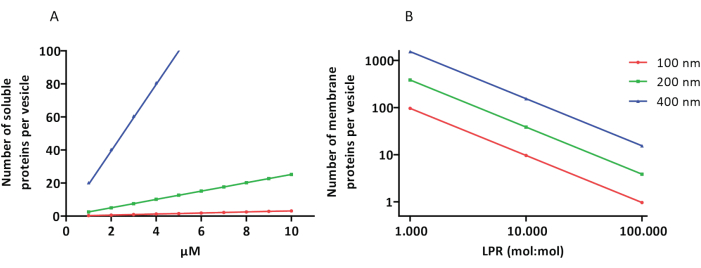
图 3:直径为 100、200 或 400 nm 的每个囊泡的分子数。 (A) 当包封的蛋白质(酶、探针)在 1-10 μM 的范围内时。 (B) 重构在每脂质 1 至 1,000、1 至 10,000 和 1 至 100,000 个膜蛋白 (mol/mol) 时进行。我们假设分子在指示的浓度下被封装,并以这些蛋白质与脂质的比例掺入膜中。对于一些酶,我们已经看到它们与膜结合,这可以增加它们在囊泡中的表观浓度。缩写: LPR = 脂质-蛋白质比率 请点击这里查看此图的较大版本.
Protocol
Representative Results
Discussion
我们提出了一种方案,用于合成含有亚微米大小脂质囊泡(proteoLUVs)的(膜)蛋白,并将proteoLUVs转化为巨型单层囊泡(proteoGUVs)。该方案应适用于其他膜蛋白13,19,30,40的重建和除此处介绍的L-精氨酸分解和甘油3-磷酸合成途径以外的代谢网络的包封。
脂质体中膜蛋白的重建通常?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
作者感谢 Aditya Iyer 克隆了 pBAD-PercevalHR 基因,并感谢 Gea Schuurman-Wolters 帮助蛋白质生产和纯化。该研究由NWO引力计划“构建合成细胞”(BaSyC)资助。
Materials
| Agarose | Sigma Aldrich | A9414-25g | |
| Amicon cut-off filter | Sigma Aldrich | Milipore centrifugal filter units Amicon Ultra | |
| BioBeads | BioRad | 152-3920 | |
| CHCl3 | Macron Fine Chemicals | MFCD00000826 | |
| D(+)-Glucose | Formedium | – | |
| D(+)-Sucrose | Formedium | – | |
| DDM | Glycon | D97002 -C | |
| Diethyl Ether | Biosolve | 52805 | |
| DMSO | Sigma-Aldrich | 276855-100ml | |
| DOPC | Avanti | 850375P-1g | |
| DOPE | Avanti | 850725P-1g | |
| DOPG | Avanti | 840475P-1g | |
| DTT | Formedium | DTT005 | |
| EtOH | J.T.Baker Avantor | MFCD00003568 | |
| Extruder | Avestin Inc | LF-1 | |
| Fluorimeter | Jasco | Spectrofluorometer FP-8300 | |
| Glycerol | BOOM | 51171608 | |
| Gravity flow column | Bio-Rad | 732-1010 | |
| Hamilton syringe 100 µL | Hamilton | 7656-01 | |
| Hamilton syringe 1000 µL | Hamilton | 81320 | |
| Handheld LCP dispenser | Art Robbins Instruments | 620-411-00 | |
| Handheld Sonicator | Hielscher Ultrasound Technology | UP50H | |
| HCl | BOOM | x76021889.1000 | |
| Imidazole | Roth | X998.4-250g | |
| K2HPO4 | Supelco | 1.05099.1000 | |
| KCl | BOOM | 76028270.1 | |
| KH2PO4 | Supelco | 1.04873.1000 | |
| Kimwipe | Kimtech Science | 7552 | |
| Large Falcon tube centrifuge | Eppendorf | Centrifuge 5810 R | |
| L-Arginine | Sigma-Aldrich | A5006-100G | |
| Light microscope | Leica | DM LS2 | |
| L-Ornithine | Roth | T204.1 | |
| LSM Laser Scanning Confocal Microscope | Zeiss | LSM 710 ConfoCor 3 | |
| MgCl2 | Sigma-Aldrich | M2670-1KG | |
| Microfluidic chip | Homemade | PDMS based | DOI: https://doi.org/10.1039/C8LC01275J |
| Na-ADP | Sigma-Aldrich | A2754-1G | |
| NaCl | Supelco | 1.06404.1000 | |
| Nanodrop Spectrometer | Isogen Life Science | ND-1000 spectrophotometer NanoDrop | |
| NaOH | Supelco | 1.06498.1000 | |
| Needles for GUVs | Henke-Ject | 14-14575 | 27 G x 3/4'' 0.4 x 20 mm |
| Needles for microfluidics | Henke-Ject | 14-15538 | 18 G x 1 1/2'' 1.2 x 40 mm |
| Ni2+ Sepharose | Cytiva | 17526802 | |
| Nigericin | Sigma-Aldrich | N7143-5MG | |
| Nutator | VWR | 83007-210 | |
| Osmolality meter | Gonotec Salmenkipp | Osmomat 3000 basic freezing point osmometer | |
| Plasmacleaner | Plasma Etch | PE-Avenger | |
| Polycarbonate filter | Cytiva Whatman | Nuclepor Track-Etch Membrane Product: 10417104 | 0.4 µm |
| Polycarbonate ultracentrifuge tube | Beckman Coulter | 355647 | |
| Pyranine | Acros Organics | H1529-1G | |
| Quartz cuvette (black) | Hellma Analytics | 108B-10-40 | |
| Sephadex G-75 resin | GE Healthcare | 17-0050-01 | |
| Sonicator | Sonics Sonics & Materials INC | Sonics vibra cell | |
| Syringe filter | Sarstedt | Filtropur S plus 0.2 | 0.2 µm |
| Syringe pump | Harvard Apparatus | A-42467 | |
| Tabletop centrifuge | Eppendorf | centrifuge 5418 | |
| Teflon spacer | Homemade | Teflon based | 45 x 26 x 1.5 or 45 x 26 x 3 or 20 x 20 x 3 mm |
| Tris | PanReac AppliChem | A1086.1000 | |
| Triton X-100 | Sigma Aldrich | T8787-100 ml | |
| Ultracentrifuge | Beckman Coulter | Optima Max-E | |
| UV lamp | Spectroline | ENB-280C/FE | |
| UV/VIS Spectrometer | Jasco | V730 spectrophotometer | |
| Valinomycin | Sigma-Aldrich | V0627-10MG | |
| Widefield fluorescence microscope | Zeiss | AxioObserver | |
| β-Casein | Sigma Aldrich | C5890-500g |
References
- Hirschi, S., Ward, T. R., Meier, W. P., Müller, D. J., Fotiadis, D. Synthetic biology: bottom-up assembly of molecular systems. Chem Rev. 122 (21), 16294-16328 (2022).
- Ivanov, I., et al. Bottom-up synthesis of artificial cells: recent highlights and future challenges. Annu Rev Chem Biomol. Eng. 12 (1), 287-308 (2021).
- Clomburg, J. M., Crumbley, A. M., Gonzalez, R. Industrial biomanufacturing: The future of chemical production. Science. 355 (6320), (2017).
- Shi, T., Han, P., You, C., Zhang, Y. -. H. P. J. An in vitro synthetic biology platform for emerging industrial biomanufacturing: Bottom-up pathway design. Synth Syst Biotechnol. 3 (3), 186-195 (2018).
- Wang, A., et al. Liver-target and glucose-responsive polymersomes toward mimicking endogenous insulin secretion with improved hepatic glucose utilization. Adv Funct Mater. 30 (13), 1910168 (2020).
- Kanter, G., et al. Cell-free production of scFv fusion proteins: an efficient approach for personalized lymphoma vaccines. Blood. 109 (8), 3393-3399 (2007).
- Zeltins, A. Construction and characterization of virus-like particles: a review. Mol Biotechnol. 53 (1), 92-107 (2013).
- Jain, K. K. Synthetic biology and personalized medicine. Med Princ Pract. 22 (3), 209-219 (2013).
- Schwille, P., Frohn, B. P. Hidden protein functions and what they may teach us. Trends Cell Biol. 32 (2), 102-109 (2022).
- Sachsenmeier, P. Industry 5.0-The relevance and implications of bionics and synthetic biology. Engineering. 2 (2), 225-229 (2016).
- Schmidt, D., Jiang, Q. -. X., MacKinnon, R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 444 (7120), 775-779 (2006).
- Godoy-Hernandez, A., et al. Rapid and highly stable membrane reconstitution by LAiR enables the study of physiological integral membrane protein functions. ACS Cent Sci. 9 (3), 494-507 (2023).
- Sikkema, H. R., et al. Gating by ionic strength and safety check by cyclic-di-AMP in the ABC transporter OpuA. Sci Adv. 6 (47), 7697 (2020).
- Foucaud, C., Poolman, B. Lactose transport system of Streptococcus thermophilus. Functional reconstitution of the protein and characterization of the kinetic mechanism of transport. J Biol Chem. 267 (31), 22087-22094 (1992).
- Yoneda, J. S., Sebinelli, H. G., Itri, R., Ciancaglini, P. Overview on solubilization and lipid reconstitution of Na,K-ATPase: enzyme kinetic and biophysical characterization. Biophys Rev. 12 (1), 49-64 (2020).
- Simidjiev, I., et al. Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc Natl Acad Sci. 97 (4), 1473-1476 (2000).
- Harris, N. J., Booth, P. J. Folding and stability of membrane transport proteins in vitro. Biochim Biophys Acta BBA – Biomembr. 1818 (4), 1055-1066 (2012).
- Jackson, M. L., Litman, B. J. Rhodopsin-egg phosphatidylcholine reconstitution by an octyl glucoside dilution procedure. Biochim Biophys Acta BBA – Biomembr. 812 (2), 369-376 (1985).
- Geertsma, E. R., Nik Mahmood, N. A. B., Schuurman-Wolters, G. K., Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat Protoc. 3 (2), 256-266 (2008).
- Rigaud, J. -. L., Pitard, B., Levy, D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim Biophys Acta BBA – Bioenerg. 1231 (3), 223-246 (1995).
- Szoka, F., Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci. 75 (9), 4194-4198 (1978).
- . Synthetic Organelles for Energy Conservation and Delivery of Building Blocks for Lipid Biosynthesis Available from: https://www.researchsquare.com/article/rs-3385355/v1 (2023)
- Lee, K. Y., et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat Biotechnol. 36 (6), 530-535 (2018).
- Méléard, P., Bagatolli, L. A., Pott, T. Giant unilamellar vesicle electroformation. Methods in Enzymology. , 161-176 (2009).
- Garten, M., Aimon, S., Bassereau, P., Toombes, G. E. S. Reconstitution of a transmembrane protein, the voltage-gated ion channel, KvAP, into giant unilamellar vesicles for microscopy and patch clamp studies. J. Vis. Exp. (95), e52281 (2015).
- Doeven, M. K., et al. lateral mobility and function of membrane proteins incorporated into giant unilamellar vesicles. Biophys J. 88 (2), 1134-1142 (2005).
- Pols, T., et al. A synthetic metabolic network for physicochemical homeostasis. Nat Commun. 10 (1), 4239 (2019).
- Bailoni, E., Poolman, B. ATP recycling fuels sustainable glycerol 3-phosphate formation in synthetic cells fed by dynamic dialysis. ACS Synth Biol. 11 (7), 2348-2360 (2022).
- Van Der Heide, T. On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J. 20 (24), 7022-7032 (2001).
- Van’T Klooster, J. S., et al. Membrane lipid requirements of the lysine transporter Lyp1 from Saccharomyces cerevisiae. J Mol Biol. 432 (14), 4023-4031 (2020).
- Lou, G., Anderluzzi, G., Woods, S., Roberts, C. W., Perrie, Y. A novel microfluidic-based approach to formulate size-tuneable large unilamellar cationic liposomes: Formulation, cellular uptake and biodistribution investigations. Eur J Pharm Biopharm. 143, 51-60 (2019).
- Weiss, M., et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat Mater. 17 (1), 89-96 (2018).
- Pols, T., Singh, S., Deelman-Driessen, C., Gaastra, B. F., Poolman, B. Enzymology of the pathway for ATP production by arginine breakdown. FEBS J. 288 (1), 293-309 (2021).
- Yandrapalli, N., Robinson, T. Ultra-high capacity microfluidic trapping of giant vesicles for high-throughput membrane studies. Lab Chip. 19 (4), 626-633 (2019).
- Elias, M., et al. Microfluidic characterization of biomimetic membrane mechanics with an on-chip micropipette. Micro Nano Eng. 8, 100064 (2020).
- Robinson, T., Kuhn, P., Eyer, K., Dittrich, P. S. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics. 7 (4), 044105 (2013).
- Cooper, A., Girish, V., Subramaniam, A. B. Osmotic Pressure Enables High-Yield Assembly of Giant Vesicles in Solutions of Physiological Ionic Strengths. Langmuir. 39 (15), 5579-5590 (2023).
- Tantama, M., Martínez-François, J. R., Mongeon, R., Yellen, G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun. 4 (1), 2550 (2013).
- Setyawati, I., et al. In vitro reconstitution of dynamically interacting integral membrane subunits of energy-coupling factor transporters. eLife. 9, e64389 (2020).
- Oropeza-Guzman, E., Ríos-Ramírez, M., Ruiz-Suárez, J. C. Leveraging the coffee ring effect for a defect-free electroformation of giant unilamellar vesicles. Langmuir. 35 (50), 16528-16535 (2019).
- Estes, D. J., Mayer, M. Electroformation of giant liposomes from spin-coated films of lipids. Colloids Surf B Biointerfaces. 42 (2), 115-123 (2005).

