Analysis of T-cell Receptor-Induced Calcium Influx in Primary Murine T-cells by Full Spectrum Flow Cytometry
Summary
Calcium influx, a measure of T-cell signaling, is an effective way to analyze responses to T-cell receptor stimulation. This protocol for multiplexing Indo-1 with panels of antibodies directed at cell surface molecules takes advantage of the highly flexible capabilities of full spectrum flow cytometry.
Abstract
Calcium influx in response to T-cell receptor stimulation is a common measure of T-cell signaling. Several calcium indicator dyes have been developed to assess calcium signaling by band-pass flow cytometry. This protocol is designed to measure calcium responses in primary murine T-cells using full spectrum flow cytometry. Total splenocytes are labeled with the ratiometric calcium indicator dye Indo-1, along with a panel of fluorochrome-conjugated antibodies to cell surface molecules. Leveraging the capabilities of full spectrum flow cytometry provides a platform for utilizing a wide array of cell surface stains in combination with Indo-1. Cells are then analyzed in real-time at 37 °C before and after the addition of an anti-CD3 antibody to stimulate the T-cell receptor. After unmixing the spectral signals, the ratio of calcium-bound to calcium-free Indo-1 is calculated and can be visualized over time for each gated population of splenocytes. This technique can allow for the simultaneous analysis of calcium responses in multiple cell populations.
Introduction
T-cell receptor (TCR) induced calcium influx is a useful measure of T-cell activation and is frequently used to determine whether a population of T-cells has impaired responses in the proximal steps of the TCR signaling pathway1. Measurements of calcium influx are generally performed by pre-labeling the T-cells with one or a pair of fluorescent calcium indicator dyes, and then examining the fluorescent signals using flow cytometry in real-time after TCR cross-linking2,3,4. Indo-1, a ratio-metric calcium dye, is excited by the UV laser with peak emissions at two different wavelengths dependent on calcium binding5, and is a commonly used indicator dye for flow cytometry analysis of calcium responses in live lymphocytes. As the emission profile of Indo-1 is quite broad, it can be challenging to combine Indo-1 assessment with simultaneous analysis of multiple cell surface markers by band-pass flow cytometry. This limitation restricts the utilization of flow cytometry analysis of calcium responses to pre-purified populations of T-cells or to populations identified by a limited set of cell surface molecules.
To address the limitations of measuring calcium responses on heterogenous populations of primary lymphocytes using band-pass flow cytometry, a protocol was developed to measure Indo-1 fluorescence using full spectrum flow cytometry. This method allows for multiplexing Indo-1 with panels of antibodies directed at cell surface molecules, taking advantage of the highly flexible capabilities of full spectrum flow cytometry. The advantage of using full spectrum flow cytometry over conventional flow cytometry is its ability to distinguish the fluorescent signals from highly overlapping dyes, thereby increasing the number of surface markers that can be simultaneously assessed in each sample. Conventional flow cytometry uses bandpass filters and is restricted to one fluorochrome per detector system6. Full spectrum flow cytometry collects signals across the entire spectrum of the fluorochrome using 64 detectors on a five-laser spectral flow cytometry system7,8. In addition, full spectrum flow cytometry takes advantage of APD (Avalanche Photo Diode) detectors that have increased sensitivity relative to photomultiplier tube detectors present on conventional flow cytometers8. Consequently, this approach is ideal for the heterogeneous cell populations, such as peripheral blood mononuclear cells or murine secondary lymphoid organ cell suspensions, as it eliminates the need for the isolation of specific T-cell populations prior to calcium dye labeling. Instead, cell surface marker expression profiles and flow cytometry gating after data collection can be used to assess calcium responses in each population of interest. As shown in this report, Indo-1 can readily be combined with eight fluorochrome-conjugated antibodies, resulting in a total of 10 unique spectral signatures. Furthermore, this method can be readily applied to mixtures of cells from congenically distinct mouse lines, allowing for the simultaneous analysis of calcium responses in wild-type T-cells compared to those from a gene-targeted mouse line.
Protocol
Mice were maintained at the University of Colorado Anschutz Medical campus in accordance with IACUC protocols. All mice were euthanized according to AAALAC standards.
1. Preparation of immune cells from the mouse spleen
NOTE: Euthanize naïve mice with CO2 euthanasia. C57BL/6 mice purchased from Jackson Laboratories and bred in-house are used for experiments at 6-12 weeks of age. Both male and female mice are utilized for experiments.
- Disinfect the mouse skin with 70% ethanol in order to reduce the possibility of external contaminants from getting into the sample.
- Dissect the mouse spleen using dissection tools, surgical scissors, and forceps. Harvest the spleen and place the detached spleen into 50 mL conical tube with ~5 mL of complete RPMI media (cRPMI) on ice.
NOTE: Complete RPMI is comprised of 10% FBS, 2 mM L-glutamine, and 1% penicillin/streptomycin.- To harvest the mouse spleen, make a 5 cm incision into the fur and skin along the left side of the mouse halfway between the front and back legs with scissors. Open the body cavity ~5 cm and remove the spleen using forceps. The spleen is the color of a kidney bean and is longer and flatter than the neighboring kidney.
- Pour the contents of step 1.2 onto a sterile 70 µm filter attached to a new 50 mL conical tube. Mechanically separate the spleen into a single cell suspension by pushing the spleen through the filter with a 5 mL syringe plunger until no spleen pulp remains on the filter surface.
- Pellet the splenocytes utilizing a swing-out rotor countertop centrifuge at 500 x g for 5 min at 4 ˚C.
- Carefully decant the supernatant. The pelleted splenocytes remain at the bottom of the 50 mL conical tube.
- To remove red blood cells, re-suspend the pelleted splenocytes in 1 mL of ACK lysis buffer for 3 min according to the manufacturer's instructions. Mix well.
- Quench the ACK lysis buffer with 15 mL of cRPMI.
- Pellet the lymphocytes as directed in step 1.4.
- Re-suspend the cell suspension in 5 mL of cRPMI in a 50 mL conical tube. Place the samples on ice.
- To count the cells, transfer 10 µL of the cell suspension to a 0.6 mL microcentrifuge tube and dilute at a 1:1 ratio with Trypan blue solution. Then, transfer to a hemocytometer or automated cell counting system.
- After counting, depending on the cell concentration in cell suspension as in step 1.9, pellet the cells in a tabletop centrifuge at 500 x g at 4 ˚C for 5 min to prepare for the addition of cRMPI media containing Indo-1 AM ester dye.
- Calculate the volume for re-suspension of the cells to achieve 10-12 x 106 cells/mL. This is a 2x concentration of the total cell count required.
- Re-suspend the cells in the volume of cRPMI calculated in step 1.11. Place the cells at 37 ˚C with 5% CO2 in either a 50 mL conical tube with the lid vented or a tissue culture plate.
2. Indo-1 ratiometric dye and fluorescent antibody labeling
- As per the manufacturer's instructions, add 50 µL of DMSO into a vial containing 50 µg of Indo-1 AM. The concentration of the stock solution is 1 µg/µL.
NOTE: DMSO is provided in micro-vials with the kit to prevent any oxidation of DMSO. - Dilute the stock aliquot (1 µg/µL) into the appropriate experimental volume (established in 1.11) of cRPMI at a concentration of 6 µg/mL, a 2x concentration. Do not store Indo-1 in an aqueous solution.
NOTE: Other buffers with added calcium can be used depending on the cell needs. - Set complete media with Indo-1 aside in a water bath at 37 ˚C.
NOTE: Use the minimum concentration of Indo-1 AM ester necessary to obtain an adequate signal. This needs to be titrated for varying cell types. Typically, a concentration between 3-5 µM is sufficient. For primary T-cells, loading cells with Indo-1 at a concentration >5 µg/mL increases mean fluorescent intensity (MFI) above a log of 6 on spectral flow cytometers. If this occurs, decrease UV laser intensity and titrate the Indo-1 to a lower concentration. - Dilute previously prepared cell suspension (in step 1.12), 1:1 in cRPMI, including the 2x Indo-1 media made in step 2.2. Ensure that the cells are at a concentration between 5-6 x 106/mL.
NOTE: Do not dye load unstained single stain control or surface marker single stain cell controls with Indo-1. Adding Indo-1 to single stain antibody controls will create a multi-color sample due to the Indo-1 added to single stained cells. Include indo-1 (calcium-free) EGTA added control to the sample plate created in step 2.6. - Add 1 mL of cells/well/experimental condition into a 12-well tissue culture plate.
- Create a single stained control for Indo-1 (calcium-free) sample using previously Indo-1 dye-loaded cells and add EGTA to a final concentration of 2 mM. EGTA will chelate calcium in the cell suspension, giving a cleaner control sample.
- Create an Indo-1 positive control (Indo-1 calcium-bound) by adding 50 µM of ionomycin to the dye loaded cell suspension at the flow cytometer. Ionomycin is a membrane permeable calcium ionophore that binds calcium ions and facilitates the transfer of calcium ions into cells.
- Create a well for biological negative control with no fluorophores or Indo-1 dye.
- Create a well for single stain controls for any additional cell populations of interest (antibodies user defined) using antibody conjugated fluorophores in a flow cytometry panel design. These will not include the Indo-1 ratiometric dye.
- Add the Fc receptor blocking antibody (~2 µg/1 x 106 cells), as per the manufacturer's instructions, in advance of adding additional fluorochrome-conjugated antibodies for surface staining.
- Incubate the plate for 45 min at 37 ˚C with 5% CO2.
- Resuspend the cells in a 12-well plate by gently tapping the plate every 15 min.
- Mix and remove the entire volume of cells suspended in the media from the 12-well plate. Then add the cell suspension to 1.7 mL microcentrifuge tubes.
- Pellet the cells in a microcentrifuge at 500 x g for 5 min at room temperature. Decant the supernatant and wash the cells by adding 1 mL of pre-warmed cRPMI. Pellet again and decant the supernatant.
NOTE: Washing the cells removes any remaining FC blocking antibody. - Resuspend the cells in 1 mL of cRPMI in 1.7 mL microcentrifuge tubes and incubate each tube at 37 ˚C for an additional 30 min at 5% CO2, leaving the top of the tube slightly open to allow for gas exchange. This allows complete de-esterification of the intracellular AM esters.
- Transfer the cells back into a 12-well tissue culture plate, if needed. Additional user defined titrated fluorochrome-conjugated antibodies can be added at a resting phase.
NOTE: Markers used in the methods panel include: Ghost 540, CD4, CD8, TCRβ, TCRγδ, CD25, and CD1d/α-galcer tetramer. Markers are chosen to analyze T-cell subsets.- Create a master mix of all the user-defined fluorochrome-conjugated antibodies according to the cell types of interest at a previously titrated volume into a 1.7 mL microcentrifuge tube.
- Add a calculated master mix for 1 mL of the cell volume, dependent on titrated antibodies to resting cells.
NOTE: The advantage of staining at step 2.15 instead of the later step 2.18 is that staining at step 2.15 will decrease the overall incubation time for the experiment. However, staining in a total volume of 1 mL at step 2.15 increases antibody usage.
- After rest, pellet the cells at 500 x g for 5 min at room temperature.
NOTE: Cells in a 12-well tissue culture plate can be pelleted in the plate with a plate centrifuge accessory. Otherwise, pellet the cells in a 1.7 mL microcentrifuge tube. - Wash the cells by resuspending the pellet in 1 mL of 4 ˚C cold cRPMI and pellet the cells again as in step 2.16. Place the cells on ice.
NOTE: If surface staining cells separately, post de-esterification at step 2.18, less antibody volume is needed. Surface staining at this step can be performed after the rest phase in cold flow buffer (1x DPBS with added 1% FBS), using user-defined antibodies, on ice for 30 min. Wash the cells after the stain in cRPMI as in step 2.17.- Add viability stain Ghost540 diluted 1:7,500 in DPBS to samples according to the manufacturer's instructions. Heat kill the cells at 55 ˚C on a warming block for single stained live/dead control.
NOTE: Viability functional dye staining to eliminate dead cells in flow cytometric analysis is performed without FBS. FBS can interact with amine dyes as per manufacturer's instructions.
- Add viability stain Ghost540 diluted 1:7,500 in DPBS to samples according to the manufacturer's instructions. Heat kill the cells at 55 ˚C on a warming block for single stained live/dead control.
- Re-suspend individual samples in 500 µL of cRPMI (phenol red-free) using a 5 mL polystyrene flow tube with a cap.
NOTE: Cells can be left at 4 ˚C for up to an hour. - Warm every 5 mL tube containing cells, individually, to 37 ˚C using a bead bath for 7 min prior to the analysis on the flow cytometer keeping time consistent between the samples. Use a timer.
3. Bead bath tube: maintaining temperature during calcium flux analysis
- Add bath beads to a small water bath, with no water added; warm up to 37 ˚C.
- Carefully cut a 50 mL conical tube in half, at the 25 mL mark, with a razor blade; discard the top of the tube.
- Fill the halved tube ¾ of the way with bath beads.
- Place the tube created in step 3.2 into the bead bath created in step 3.1. Bring the tube and beads to 37 ˚C.
- Plug the bead bath into an electrical outlet next to the flow cytometer in order to maintain the temperature in preparation for sample analysis.
- Add a 50 mL conical Styrofoam rack cut to hold one tube to position the tube in place while running on the flow cytometer. This will eliminate the need to manually hold the tube for the duration of the curve analysis.
4. Acquisition of calcium influx using flow cytometric analysis
- Log into the flow cytometer software on a flow analyzer.
- Click on the Library tab to add Indo-1 (calcium-bound) and Indo-1 (calcium-free) fluorescent tags. Select Fluorescent Tags on the software menu on the left. Select UV laser under fluorescent tag groups and click on +add to add a fluorescent tag name (Indo-1 (calcium-bound)/Indo-1 (calcium-free)) to the library. Choose the laser excitation wavelength and the emission wavelength, and then click on Save. Continue to the experimental setup.
NOTE: Indo-1 is excited by the UV laser at a wavelength of 355 nm. The emission wavelength of Indo-1 (calcium-bound) is 372 nm. The emission wavelength of Indo-1 (calcium-free) is 514 nm. - Click on the Acquisition tab. Open/create a new experiment and add the desired fluorescent tags to the experiment.
- Create Reference Group and a new sample group/s for the experiment. Label both the samples and the reference groups as necessary.
- Under the Acquisitions tab, set the events to record to the maximum: 10,000,000.
- Set the stopping volume to the maximum volume: 3,000 µL.
- Set the stopping time to the maximum time: 36,000 s.
- Acquire single stained controls for defined conjugated-antibodies included in the multicolor panel.
- Acquire single stained controls for Indo-1 functional dye.
- Add 50 µM of ionomycin to the calcium bound Indo-1 positive control. Vortex to mix. Add the flow tube to the sample injection port and click on Record.
- Add calcium free Indo-1 negative control to the flow cytometer and click on Record. EGTA was added in step 2.6.
- Unmix the single stained controls by clicking on the Unmix button.
NOTE: All the single stained controls from all the conjugated antibodies and functional dyes must be recorded prior to unmixing the samples. For the Unmix button to function, all the reference controls need to be recorded first. Unmixing can be done before or after sample collection.
- Once unmixing is complete, create sequential plots using the unmixed worksheet. SSC-A versus FSC-A removing debris from sample, SSC-A versus SSC-H to remove doublets. This is followed by a viability clean-up gate along with further gating on populations of interest. To create gates, click on Plot. Add a plot to the worksheet, and double click inside the gate to create a downstream gated population. Continue until all the populations of interest are accounted for.
- Warm single stained control samples (from steps 2.6-2.9 in section Indo-1 ratiometric dye and fluorescent antibody labeling) to 37 ˚C for sequential analysis. Set up the flow cytometry software.
- Run DI water on the cytometer for 2-3 min to ensure the fluidic stability of the flow cytometer.
- Set up sequential plots on the unmixed worksheet by creating gates using the Polygon, Rectangle or Ellipse gate buttons. Gate to include the population of interest (i.e., lymphocytes using SSC-A vs. FSC-A [size/lymphocytes discrimination]). Negative populations appear clustered around zero whereas positive populations can be visualized by increasing MFI. Populations of interest will be user defined based on the biological question.
- Double click inside the gate created and change Y-axis and X-axis parameters to SSC-A versus SSC-H (singlet discrimination). Complete defining the axis parameters by left clicking on the words on the axis.
- Create a plot with SSC-A versus viability dye signal (viability gate parameters). Gate on live cells, which are negative for amine dye staining. Live cells, negative for live dead staining, will cluster at the 0 mark on both the Y and X axes.
- Double click on the inside plot to create a new plot containing only single cell live lymphocytes. Change the Y-axis to Indo-1 (bound-calcium) (V1) and/or Indo-1 (free-calcium) (V7) versus time on the X-axis for visualization of the calcium influx.
- Place the warmed biological sample onto the machine SIT and run the samples at 2,500-3,000 events/s on medium to allow visualization of calcium influx in limited cell number populations (e.g., iNKT).
NOTE: By resuspending the cells at a concentration of 1 x 106/mL, the cell events collected at medium flow rate should equal 2,500-3,000 events/s. Lower the flow rate under acquisition control by clicking on the drop-down menu or dilute the sample if the cell suspension has an increased concentration. Running the samples at a high flow rate can increase the spreading of the signal from one fluorophore to other fluorophores in the panel. - Add the next tube to the bead bath to warm to 37 ˚C.
- In the Acquisition Control, press Record to record the initial data for 30 s to obtain a basal level of calcium signaling in the sample.
- In the Acquisition Control, click on Stop and remove the tube from Sample Injection Port (SIP).
- Add 30 µg of unconjugated anti-CD3 directly into the sample tube to initiate calcium influx. The final concentration per tube is 60 µg/mL.
NOTE: There is no washing step after the addition of anti-CD3. - Place the tube back on machine SIP as quickly as possible and press Record in the software.
- Record data for 7 min, watching the time elapsed under the acquisition controls in the software, to observe the time-course of calcium influx within the sample populations.
- After 7 min, press Stop in the software and add 1 µg/mL of ionomycin. Record for 30 s to obtain the maximum calcium signal in the cells of interest. To limit carryover of ionomycin into the next sample, complete two SIT flushes on the instrument between samples.
- Continue to the next sample and repeat the workflow until all the samples have been collected.
- Save the experiment and click on the export zip file. Unmixed files (.fsc) are uploaded to external flow cytometry analysis software.
5. Calcium curve analysis
- Using an analysis software6 for flow cytometry, convert Indo-1 fluorescent signals to a ratio for time-lapse calcium measurements. Derive parameters under the tools tab by inserting references Indo-1 (calcium-bound)/Indo-1 (free-calcium) using a linear scale. Set the minimum to zero and the maximum according to the influx of calcium ratio, typically around 10. The maximum ratio varies by cell type and MFI of Indo-1.
- Highlight the selected parameter. Under tools, add kinetic analysis to choose the parameter by clicking on the Kinetics tab. Set the Y-axis to derived.
- Select MFI (mean fluorescent intensity) within the Kinetics tab.
- Add Gaussian smoothing, if desired. To add Gaussian smoothing, select the option in the pull-down statistics menu in the flow analysis software. Gaussian smoothing can be added to smooth the visualization of the calcium influx curve of analysis.
- Create multiple ranges for statistics along the calcium flux time course for biological comparisons within the samples.
- Drag the samples into the layout editor and add the statistics as desired. Statistical analysis varies depending on the biological question. For publication purposes, multiple replicates are analyzed using t-test or ANOVA.
- For a moment of time analysis, set the gate on a specific moment in time along the calcium flux. Examine the desired surface markers and gate on the population of interest; then, assess a two-dimensional dot-plot of Indo-1 (calcium-free) versus Indo-1 (calcium bound) for gated cells within that moment in the time region.
Representative Results
The experimental workflow to assess calcium responses in primary murine T-cells using an Indo-1 ratiometric dye with multiplexing surface stains is shown in Figure 1. After harvesting and processing the mouse splenocytes into a single cell suspension, the cells are stained with an Indo-1 AM ester and surface stained with fluorochrome-linked antibodies. Once the dye loading and antibody staining are completed, the lymphocyte samples are warmed up to a biological temperature (37 °C). The samples are then analyzed using full spectrum flow cytometry for Indo-1 fluorescence after gating on the individual cell types desired, without the need for sorting the samples prior to analysis. In order to use Avalanche Photo Diode (APD) detectors within the full spectrum flow cytometer, it is necessary to convert the peak emission wavelength from a bandpass filter wavelength measured in nm to its respective channel on the APD. The table shown in Figure 2 provides a guideline, but the optimum detectors for the two fluorescence signatures of Indo-1 (calcium-bound versus calcium-free) must be determined empirically. This is accomplished by preparing single stained Indo-1 samples using Ionomycin to generate the Indo-1 (calcium-bound) signal, and EGTA, a calcium chelating agent, to generate the Indo-1 (calcium-free) signal. After analysis using full spectrum flow cytometry, the channel displaying the peak fluorescence intensity for each Indo-1 signature can be visualized (Figure 3A). By normalizing the MFI of the positive populations to that of the negative populations, the shift in Indo-1 fluorescence from calcium-free to calcium-bound can be determined (Figure 3B). This display is generated in a workbook (.xls) using reference controls for both bound and free Indo-1, drawing a negative and positive interval gate with clear positive and negative gates for MFI normalization as shown in (Figure 3A). Sample MFI statistics can also be obtained by right clicking on the stats table. In the software, determine the MFI ratio by dividing the positive population with the negative population and overlay the two spectra on a single display (Figure 3B, right). The yellow dotted arrow shows the normalized spectral differences between the spectral signatures of both bound and free Indo-1.
To evaluate calcium responses following T-cell receptor stimulation, the Indo-1 fluorescence is assessed after gating on the cell populations of interest, as shown in Figure 4. First, a plot of time versus side scatter (SSC-A) is examined to assess fluidic stability; a stable consistent signal across the time axis indicates stability (Figure 4A). Next, the forward versus side scatter (FSC-A vs. SSC-A) is visualized, and the lymphocyte population is gated as shown in (Figure 4B). After the lymphocyte gate, a single cell discrimination gate is applied by generating a plot of side scatter height versus area (SSC-H vs. SSC-A), and the singlets, which fall on the diagonal, are gated as shown in Figure 4C. The singlets are then assessed for viability, by examining staining with the viability dye and gating on the negative population (Figure 4D). Finally, to analyze T-cells, a B-cell dump/internal negative control gate is applied by gating on the CD19-negative population (Figure 4E). The CD19-negative population is then assessed for T-cell subsets using antibodies to CD4 and CD8 (Figure 4F); the example shown examines CD4+ T-cells for calcium responses by visualizing Indo-1 (calcium-bound) fluorescence versus time (Figure 4G). After gating on a time segment, the Indo-1 (calcium-free) versus Indo-1 (calcium-bound) plot can also be generated (Figure 4H). This analysis will later be used to derive the Indo-1 ratio (see methods calcium influx curve analysis in flow cytometry analysis software).
At this point in the analysis, it may be useful to analyze moments in time within the two-dimensional plot of Indo-1 (calcium-bound) versus time in individual cell populations of interest. The optimal timepoints include the baseline calcium bound to Indo-1 prior to TCR stimulation, the peak response, a timepoint several minutes after the peak response as intracellular calcium levels decline, and finally the calcium response elicited by adding ionomycin (Figure 5A). In the baseline sample prior to anti-CD3 antibody addition, a weak Indo-1 (calcium-bound) signal is detected. During the peak response, the cells show a strong Indo- 1(calcium-bound) signal and a reduced fluorescence of Indo-1 (calcium-free). At 5 min post-peak, the Indo-1 fluorescence nearly returned to baseline. After ionomycin is added to the sample, a robust signal of Indo-1 (calcium-bound) can be visualized, ensuring adequate dye-loading of the cells. Rare cell populations (<1 %) can be difficult for derivative analysis; to overcome this limitation, analysis of the rare populations can be performed by plotting Indo-1 (calcium-bound) versus Indo-1 (calcium free) after gating on each population of interest, such as CD4+ cells, TCRb+ cells, TCRyg+ cells, CD25+ cells, iNKT cells, and CD19+ cells (Figure 5B,C). Note the readily detectable calcium response in TCRγδ+ T-cells and iNKT cells (CD1d/α-galcer+). For comparison of surface proteins within a mixed murine T-cell population, two-dimensional plots were created showing subsets of interest. Below, the analysis of the calcium response over the entire time course for each subset is displayed (Figure 5C).
This assay can also be used to establish biological differences in T-cells from wild-type versus gene-targeted mice or in T-cells untreated versus treated with pharmacological inhibitors. In the example shown in Figure 6, T-cells were treated with PRN694, a small molecule inhibitor of the T-cell tyrosine kinases, ITK and RLK9,10. Inhibition of ITK/RLK dampens T-cell receptor signaling, leading to a reduced calcium response following T-cell receptor stimulation11 (Figure 6A). To quantify the effects of ITK/RLK inhibition, the curves displaying the ratio of Indo-1 (calcium-bound) to Indo-1 (calcium-free) fluorescence can then be analyzed for the area under the curve (AUC) or slope of the curve for each condition (Figure 6B,C). This quantification is performed by sectioning the time course of the calcium response into discrete segments (Figure 6A), and assessing the area under the curve or the slope of the curve for each segment, as shown (Figure 6B,C). Overall, this protocol demonstrates that full spectrum flow cytometry can be used to measure calcium influx along with multiple cell surface markers in immune cell populations under investigation. These results show that cell subsets represented at greater than 1% of the total population can be assessed for calcium influx over time. In contrast, less abundant cell subsets require alternative analysis using moments of time.
Statistical analysis of calcium response data is specific to the experimental question and the biological assays that are being performed. In the data presented in the manuscript, experiments were performed with replicates to ensure experimental accuracy and the robustness of the methodology; however, multiple experiments on different biological samples over different days were not performed. Therefore, it would not be appropriate to perform statistical analyses on the data presented.
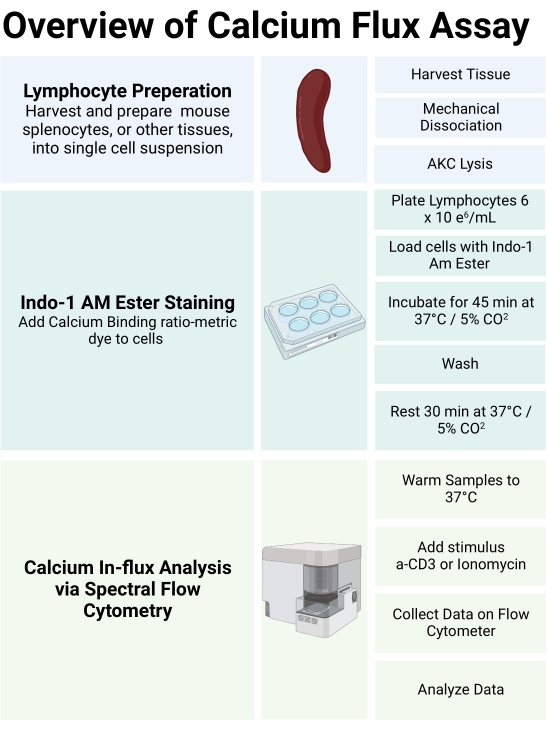
Figure 1: Schematic of calcium assay workflow. The use of the Indo-1 AM ester calcium indicator dye provides a tool to visualize the influx of calcium in immune cells and their sub-populations. This schematic outlines the workflow for analysis of calcium responses in murine splenic T-cells. Please click here to view a larger version of this figure.
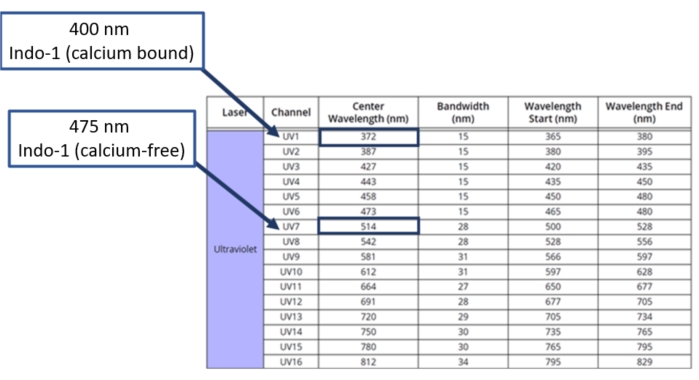
Figure 2: Conversion of PMT wavelengths to APD channels. The full spectrum flow cytometer has 16 detectors to detect fluorescent emissions following excitation by the UV laser. The specifications of each detector are indicated in the table. Highlighted are the two detectors used for the assessment of Indo-1 fluorescence, as were determined empirically using single stained controls. Conversions to APD wavelength and user manual for spectral flow cytometer are available11. Please click here to view a larger version of this figure.
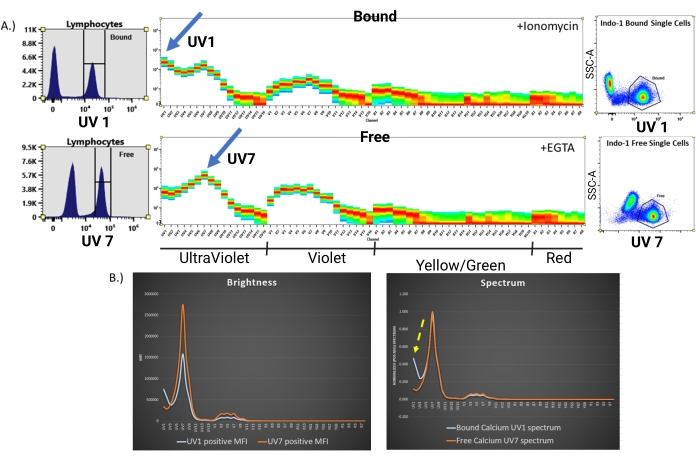
Figure 3: Visualization of the Indo-1 spectral signature. (A) Top: Spectral signature of Indo-1 fluorescence in CD8+ T-cells following ionomycin introduction to elicit a robust influx of calcium. The peak of Indo-1 (calcium-bound) can be visualized in UV1. Bottom: Spectral signature of Indo-1 (calcium-free) showing the peak fluorescence in UV7. This control sample was generated by treating Indo-1-loaded cells with EGTA12 to chelate any extracellular free calcium. (B) Combined five laser spectrum overlay of the Indo-1 signature, showing raw spectral MFI on the left and the normalized spectra of Indo-1 bound versus free Indo-1 on the right. Visualization of the shift from Indo-1 (calcium-free) in orange to Indo-1 (calcium-bound) in blue can be readily detected. Please click here to view a larger version of this figure.
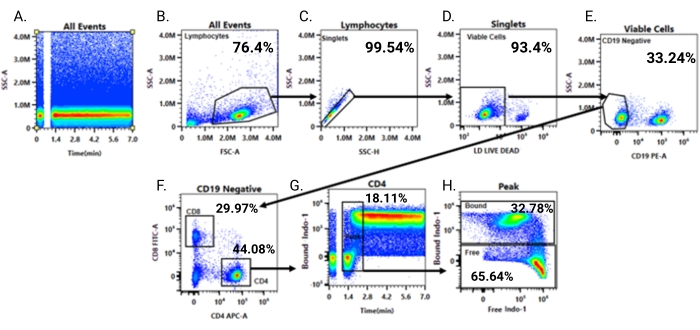
Figure 4: Gating strategy for visualization of calcium responses. The workflow for sequential gating of the samples is outlined. (A) Fluidic stability is examined using a time versus SSC-A gate. (B) FSC-A versus SSC-A is used to gate on lymphocytes, thereby eliminating cell debris and residual red blood cells in the sample. (C) Single cells are gated on using a SSC-H versus SSC-A plot. (D). The singlet gate is then applied to a plot of live-dead Ghost540 versus SSC-A; gating on Ghost540-negative cells eliminate non-viable cells from subsequent analysis. (E) Live cells are analyzed for CD19 versus SSC-A, and CD19-negative cells (non-B cells) are gated on. (F) The CD19-negative cells are examined for CD4 versus CD8 staining. (G) CD4+ cells are then gated on to visualize time versus Indo-1 (calcium-bound). (H) A moment in time is selected to represent the initiation of the calcium influx response following anti-CD3 antibody addition by visualizing Indo-1 (calcium-free) versus Indo-1 (calcium-bound) fluorescence. Please click here to view a larger version of this figure.
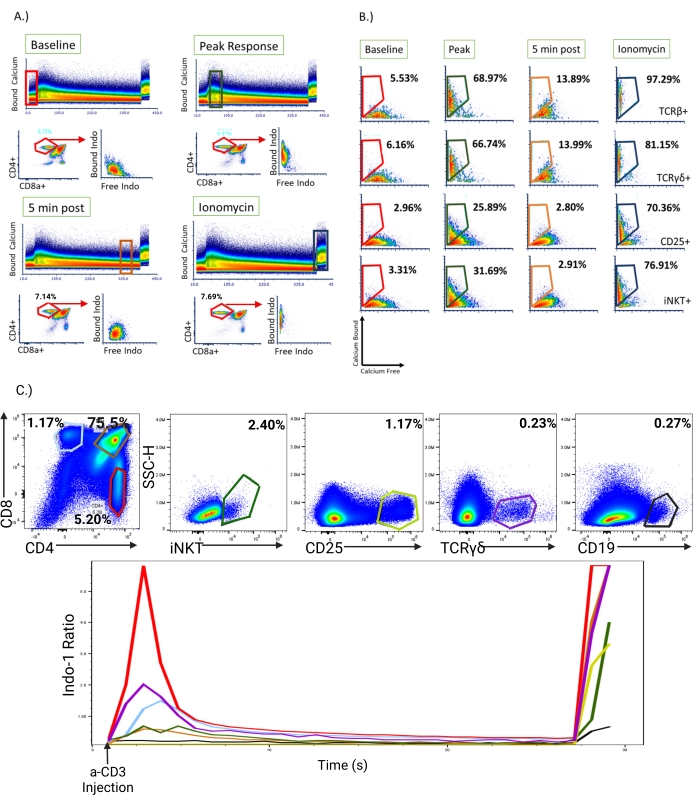
Figure 5: Visualization of Indo-1 fluorescence in gated populations of murine thymocytes at discrete timepoints in the calcium response. Total thymocytes were stimulated with anti-CD3 antibody followed by ionomycin after 6 min of the sample collection. At four discrete timepoints, as indicated in the rectangular boxes outlined on the two-dimensional plots of time versus Indo-1 (calcium-bound), different subpopulations of thymocytes were examined for calcium responses. (A) Two-dimensional plots showing gating for the baseline Indo-1 signal, the peak response, 5 min post-peak response, and the response to ionomycin. Below each plot CD4 versus CD8 staining (left) and the plot of Indo-1 (calcium-free) versus Indo-1 (calcium-bound) on gated CD4 single positive (SP) thymocytes is shown. (B) Plots of Indo-1 (calcium-free) versus Indo-1 (calcium-bound) on gated subsets of thymocytes are shown at each of the four time-points, using the gating strategy shown in (A). (C) Two-dimensional plots were created showing staining of total thymocytes with the indicated antibodies. Specific subsets were gated (top row) on and examined for calcium responses versus time (bottom). Light blue (SP) CD8+ population, red (SP) CD4 population, which exhibits the greatest influx of calcium, orange CD4+CD8+ double-positive thymocytes, dark green iNKT cells, light green CD25+, purple TCRγδ+ T-cells, and black CD19+ B cells. Please click here to view a larger version of this figure.
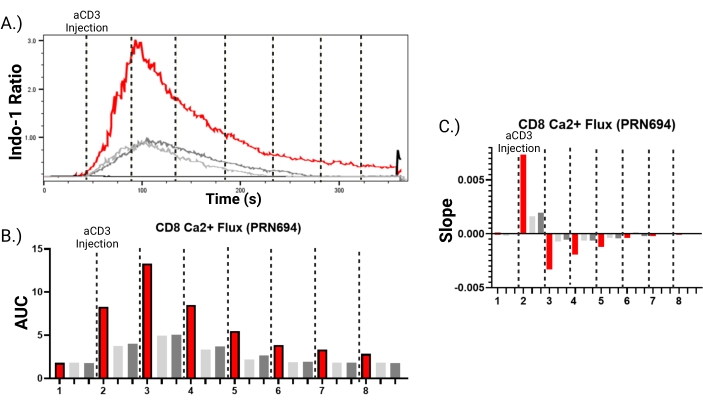
Figure 6: Inhibition of the calcium response in CD8+ T-cells treated with a small molecule inhibitor of ITK/RLK. During the Indo-1 dye-loading, splenocytes were treated with two doses of PRN694, 100 nM (dark gray) and 200 nM (light gray). (A) Indo-1 ratio (calcium-bound/calcium-free) is shown versus time for untreated cells (red) and cells treated with PRN694. Curves show calcium response of gated CD8+ T-cells. The black line represents the negative control of splenocytes loaded with Indo-1 and surface stained, but not stimulated with anti-CD3 antibody. (B,C) The data can be analyzed by dividing the time axis shown in (A) into eight segments, visualized with black dotted lines, and calculating the area under the curve (AUC) (B), or the slope of each curve (slope) (C) at each time interval of the response. Please click here to view a larger version of this figure.
Discussion
This protocol describes an optimized assay designed to measure calcium responses in primary murine T-cells loaded with titrated Indo-1 ratiometric indicator dye using full spectrum flow cytometry7,8. The advantage of performing calcium flux assays using full spectrum flow cytometry is the ability to multiplex surface cell marker staining in combination with assessment of Indo-1 fluorescence. Full spectrum flow cytometry has the advantage of allowing the use of highly overlapping dyes, thereby increasing the number of markers that can be used in a panel. This is due to the fact that the full spectrum flow cytometer collects all of the laser light emitted across an array of detectors for each sample analyzed, rather than having one detector dedicated to one fluorochrome. Using the entire spectrum of each fluor allows for higher sensitivity of the assay and provides a means to identify spectrally unique fluorochromes that would have overlapping signals if collected on a band-pass flow cytometer. This also eliminates the need for pre-isolation of a cell subset under investigation.
In this study, a panel of cell surface markers were assessed with fluorochrome-conjugated antibodies. This panel included αCD4-APC-Cy7, αCD8-FITC, αCD19-PE, αTCRβ-PerCP-Cy5.5, α-TCRδ-PE-Cy5, αCD25-PE-Cy7, and CD1d-αgal-cer tetramer-APC; in addition, the panel included the live-dead Ghost540 dye. The optimal channel to detect each of these fluorochromes is available in the full spectrum flow cytometer users manual13. In contrast, the detection of Indo-1 (calcium-free) and Indo-1 (calcium bound) was empirically determined, although an approximate peak channel for detection of each fluorescent signature was estimated based on the known peak emission wavelengths for the two forms of Indo-1. It is also possible to convert the peak emission wavelengths from bandpass filter wavelengths used on conventional flow cytometers and measured in nm to their respective channels on the Avalanche Photo Diode (APD) detectors used by the full spectrum flow cytometer; this can also provide an estimate of optimal channels to detect Indo-1 (calcium-free) and Indo-1 (calcium bound), respectively. Since single stained controls are included for each fluorescent tag, the unmixing process deconvolutes the fluorescent signatures, allowing visualization of each fluorochrome's intensity on cells in each sample. As described in this method, data analysis can be performed by gating on cell subsets of interest and visualizing the ratio of detect Indo-1 (calcium-free) and Indo-1 (calcium bound) versus time.
There are limitations of this assay. For instance, concentrations of Indo-1 below 3 µM did not allow for successful visualization of the calcium influx response in T-cells. As this assay also accommodates multiplexing measurements of the calcium response with analysis of multiple cell surface markers on heterogeneous populations of cells using full spectrum flow cytometry, it is imperative to carefully titrate all fluorescently tagged antibodies used, including those used for the single stained controls. This is important for successful implementation of the highly sensitive linear unmixing algorithm14. In addition, a complete time course of calcium influx could not be visualized for cell subsets representing less than 1% of the total population being analyzed. Instead, an assessment of the calcium response in these rare subsets required an alternative analysis strategy using moments in time15. The moments in time analysis provides snapshots of the fluorescent signals of Indo-1 (calcium-free) versus Indo-1 (calcium bound) at discrete stages of the calcium response, rather than the entire time course of the response. Finally, it is also important to recognize the limitations of using single surface markers to define subsets of T-cells. For instance, staining thymocytes with α-CD25 antibody is not sufficient to distinguish CD4+ regulatory T-cells from all other thymocyte populations. This is due to the fact that the majority of early thymocyte progenitor (CD4-CD8-) cells also express CD2516. This is likely to account for the absence of a detectable calcium influx response in gated CD4+CD25+ thymocytes.
The optimization of this protocol required careful assessment of several variables that were key to assay success. These include optimization of the specific clone of anti-CD3 antibody used, titration of the optimal concentration of this antibody, and the need for antibody crosslinking using the traditional biotin/streptavidin system. For murine T-cells, the concentration of 30 µg/sample for 6 x 106 cells in a 500 µL volume was found to be the minimum amount of antibody necessary for optimal calcium responses. Interestingly, robust calcium responses were observed with anti-CD3 antibody clone 17A2, but not with clone 145-2C11; furthermore, when using clone 17A2, adding a secondary crosslinking reagent was unnecessary17. Tests using biotinylated anti-CD3 clone 17A2 with or without streptavidin showed no enhancement of the calcium response in the presence of streptavidin crosslinking. It is possible that this antibody included aggregates, and that these aggregates accounted for the stimulation efficacy of the antibody in the absence of overt cross-linking. However, as these experiments were performed over the course of many months with different vials of this anti-CD3 antibody, the results obtained were highly reproducible; therefore, the ability of this antibody to stimulate T-cells is not likely to vary among users.
Robust calcium responses in primary T-cells require maintaining the cells at the biological temperature of 37 ˚C throughout the sample acquisition; in contrast, primary B cells will show a calcium response to anti-IgM antibody at room temperature. Based on the sensitivity of the T-cell calcium response to temperature, it is critical to ensure that each sample is treated the same; for instance, each sample must be warmed to 37 ˚C using the same method and for the same length of time. In the absence of this consistency, variations in calcium responses may be observed, but may not be indicative of biologically relevant differences between samples.
In summary, this protocol describes the details of performing calcium flux assays based on the assessment of Indo-1 fluorescence using full spectrum flow cytometry in combination with multiplex surface cell marker staining. The method allows investigators to avoid the need for isolation or sorting of specific cell populations prior to calcium dye labeling. Furthermore, this protocol outlines an additional analysis methodology used to visualize calcium responses at discrete moments in time within distinct cell subpopulations. The moments in time analysis can be successfully applied to rare (<1% of total) cell populations, which are otherwise undetectable when assessing the entirety of the calcium response curve. In future, this assay could be expanded to the use of additional fluorochrome-conjugated antibodies, taking advantage of the extensive capabilities of the full spectrum flow cytometer.
Disclosures
The authors have nothing to disclose.
Acknowledgements
R01AI132419, CU | AMC ImmunoMicro Flow Cytometry Shared Resource, RRID:SCR_021321, Many thanks to our colleagues at Cytek for continual discussions of full spectral cytometric analysis on the Aurora and SpectroFlo software. Figures were created with BioRender.com.
Materials
| 12 well TC treated plates | Cell Treat | 229111 | |
| 50 mL conical | Greiner Bio1 | 41-12-17-03 | 50 mL Polypropylene centrifuge tubes with cap |
| 5mL polysterene flow tubes | Corning | 352052 | |
| 5mL syringe | BD syringe | 309646 | plunger only is used sheith is discarded |
| 70uM filter | Greiner bio1 | 542070 | |
| aCD3 (17A2) | Biolegend | 100202 | |
| AKC lysis Buffer | Gibco | A1049201 | |
| Aurora Spectral Flowcytometer | https://cytekbio.com/pages/aurora | ||
| Bath Beads | coleparmer | Item # UX-06274-52 | |
| CD19 PE | Tonbo | 50-0193-U100 | |
| CD1d Tetramer APC | NIH | ||
| CD25 PECy7 | ebioscience | 15-0251 | |
| CD4 APC Cy7 | Tonbo | 25-0042-U100 | |
| CD8a FITC | ebioscience | 11-0081-85 | |
| Cell Incubator | Formal Scientific | ||
| Dissection Tools forceps | McKesson | #487593 | Tissue Forceps McKesson Adson 4-3/4 Inch Length Office Grade Stainless Steel NonSterile NonLocking Thumb Handle 1 X 2 Teeth |
| Dissection Tools Scissors | McKesson | #970135 | Operating Scissors McKesson Argent™ 4-1/2 Inch Surgical Grade Stainless Steel Finger Ring Handle Straight Sharp Tip / Sharp Tip |
| DPBS 1x | Gibco | 14190-136 | DPBS |
| EGTA | Fisher | NC1280093 | |
| FBS | Hyclond | SH30071.03 | lot AE29165301 |
| FlowJo Software | https://www.flowjo.com/ | ||
| Indo1-AM Ester Dye | ebioscience | 65-085-39 | Calcium Loading Dye |
| ionomycin | Millipore | 407951-1mg | |
| Live/Dead Ghost 540 | Tonbo | 13-0879-T100 | |
| Microcentrifuge tubes 1.7mL | Light Labs | A-7001 | |
| Penicillin/Streptomycin/L-Glutamine | Gibco | 10378-016 | |
| PRN694 | Med Chem Express | Hy-12688 | |
| Purified Anti-Mouse CD16/CD32 (FC Shield) (2.4G2) | Tonbo | 70-0161-M001 | FC Block |
| RPMI | Gibco | 1875093 | + phenol red |
| RPMI phenol free | Gibco | 11835030 | -phenol red |
| Table top centrifuge | Beckman Coulter | Allegra612 | |
| TCRβ PerCP Cy5.5 | ebioscience | 45-5961-82 | |
| TCRγ/δ Pe Cy5 | ebioscience | 15-5961-82 | |
| Vi-Cell Blu Reagent Pack | Product No: C06019 | Includes Tripan | |
| Vi-Cell Blu | Beckman Coulter | ||
| Waterbath | Fisher Brand Dry bath |
References
- Weiss, A., Imboden, J., Shoback, D., Stobo, J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proceedings of the National Academy of Sciences of the United States of America. 81 (13), 4169-4173 (1984).
- Huang, G. N. Cell calcium mobilization study (flow cytometry). Bio-Protocol. 2 (9), 171 (2012).
- June, C. H., Moore, J. S. Measurement of intracellular ions by flow cytometry. Current Protocols in Immunology. , (2004).
- Posey, A. D., Kawalekar, O. U., June, C. H. Measurement of intracellular ions by flow cytometry. Current Protocols in Cytometry. 72, 1-21 (2015).
- Grynkiewicz, G., Poenie, M., Tsien, R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. The Journal of Biological Chemistry. 260 (6), 3440-3450 (1985).
- McKinnon, K. M. Flow cytometry: An overview. Current Protocols in Immunology. 120, 1-11 (2018).
- Nolan, J. P., Condello, D. Spectral flow cytometry. Current Protocols in Cytometry. , (2013).
- Bonilla, D. L., Reinin, G., Chua, E. Full spectrum flow cytometry as a powerful technology for cancer immunotherapy research. Frontiers in Molecular Biosciences. 7, 612801 (2021).
- Zhong, Y., et al. Targeting Interleukin-2-inducible T-cell Kinase (ITK) and Resting Lymphocyte Kinase (RLK) using a novel covalent inhibitor PRN694. The Journal of Biological Chemistry. 290 (10), 5960-5978 (2015).
- Gallagher, M. P., et al. Hierarchy of signaling thresholds downstream of the T-cell receptor and the Tec kinase ITK. Proceedings of the National Academy of Sciences of the United States of America. 118 (35), 2025825118 (2021).
- Andreotti, A. H., Schwartzberg, P. L., Joseph, R. E., Berg, L. J. T-Cell signaling regulated by the tec family kinase, Itk. Cold Spring Harbor Perspectives in Biology. 2 (7), 002287 (2010).
- Nakamura, Y. EGTA can inhibit vesicular release in the nanodomain of single Ca2+ channels. Frontiers in Synaptic Neurosciene. 11, 26 (2019).
- Cytek Aurora Users Guide. Cytek Available from: https://depts.washington.edu/flowlab/Cell%20Analysis%20Facility/Aurora%20User%20Guide.pdf (2022)
- SpctroFlo Software. Cytek Available from: https://cytekbio.com/pages/spectro-flo (2022)
- FlowJo Software. Becton-Dickinson Available from: https://www.flowjo.com/solutions/flowjo (2022)
- Godfrey, D. I., Zlotnik, A. Control points in early T-cell development. Immunology Today. 14 (11), 547-553 (1993).
- Vossen, A. C., et al. Fc receptor binding of anti-CD3 monoclonal antibodies is not essential for immunosuppression, but triggers cytokine-related side effects. European Journal of Immunology. 25 (6), 1492-1496 (1995).

