Channelrhodopsin2 Mediated Stimulation of Synaptic Potentials at Drosophila Neuromuscular Junctions
Summary
This procedure uses a blue light-activated algal channel and cell-specific genetic expression tools to evoke synaptic potentials with light pulses at the neuromuscular junction (NMJ) in Drosophila larvae. This technique is an inexpensive and easy-to-use alternative to suction electrode stimulation for synaptic physiology studies in research and teaching laboratories.
Abstract
Protocol
Part 1: Animal care and genetic crosses
- Maintain UAS-ChR2 and OK371-GAL4 fly lines in separate bottles containing standard fly media 8.
- Collect virgins of OK371-GAL4 fly lines and males of UAS-ChR2 lines.
- Put both males and females in a vial containing standard fly media mixed with 1 mM all-trans retinal (ATR). ATR food should be made by first melting regular fly media in a microwave for ~1 minute. Once melted, allow to cool for about 30 seconds to one minute and then add 100 μl of 100 mM ATR in 100% ETOH for every 10 ml of fly media. Place vials on ice, then store in a dark area at 4°C.
- Let flies mate and lay eggs in a dark area at 22-25°C.
- Wait 3-4 days until 3rd instar larvae are visible; at that point, dispose of adult flies.
Part 2: Rig setup
- Attach any 10x dissecting scope eye piece (in our case, from Carl Zeiss Inc., www.zeiss.com, Thornwood, NY), to blue LED light source with heat sink (Thor labs, www.thorlabs.com, Newton, NJ). Attach to magnetic base with post and clamp. Cover heat sink with an electrically grounded shield to reduce electrical noise generated by the light source.
- Place magnetic base with light source on air table of electrophysiology rig.
- Connect light source to control circuit and connect control circuit to external voltage source (Powerlab 4/30, ADInstruments, www.adinstruments.com, Colorado Springs, CO).
- Give 1-5 V pulses to control circuit to activate blue light.
- Adjust magnetic base and light source until blue light beam is focused on area to be occupied by larval dissection.
Part 3: Dissection
- Place six 0.1 mm insect pins into the floor of a sylgard-lined dish.
- Remove a 3rd instar larvae from food media and place into any plastic Petri-dish.
- Rinse larvae with saline to remove food.
- Place larvae in dissecting dish near the pulled pins and add saline to ½ level.
- Orient the larvae so that you can see two silvery tubes (trachea) running along the animal’s dorsal surface.
- Insert a large pin directly into the tail in between the tracheal tubes. Hold the larvae down and place a second large pin into its head, being sure to stretch the body out lengthwise.
- Make a small incision near the tail, and continue it up the length of the body. Make sure that the tips of the scissors are raised so as not to accidentally cut ventral nerves and/or body wall muscles.
- Place four pins on the four corners of the animal’s now open midsection. Set them into the dissecting dish so as to fillet the animal. Pin preparation out taught.
- Using forceps and scissors, remove the animal’s guts, trachea and fat bodies.
- Rinse the prep with saline.
- Locate the frontal lobes (as seen in Fig A) and ventral ganglion of the prep.
- Using micro-scissors, carefully cut through the ventral ganglion just behind the frontal lobes.
Part 4: Muscle recording and blue light stimulation
- Pull a 10-20 μω electrode using an electronic electrode puller (Sutter Instruments).
- Fill the pulled electrode with 3 M KCl.
- Place filled electrode in electrode holder and maneuver electrode tip with a micromanipulator near larval prep under dissecting microscope on electrophysiology rig.
- Identify Muscle 6 (M6) in any body wall segment (Fig A).
- Carefully insert electrode into M6 and watch for rapid hyperpolarization. Muscle resting membrane potentials should be anywhere from -30 mV to -70 mV.
- Adjust blue light so that dissected prep is centered in light beam.
- Give 20-100 ms voltage pulses to control circuit. Incrementally increase voltage supplied to control circuit. Watch for excitatory junction potentials in the muscle cell (Figure 1B).
Representative Results:
Figure 1A shows a schematic of the recording setup and filleted preparation. Figure 1B shows typical EJP evoked by short light pulses. EJP amplitude shown is summed amplitude from two motor neurons known to both innervate M6. Lower light intensities only activated one motor unit (data not shown).
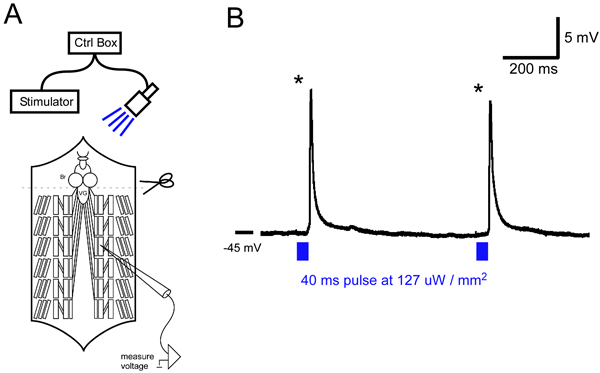
Figure 1: A) General schematic of an intracellular recording rig and with blue LED. Brain (Br) is removed to inhibit rhythmic activity in the ventral ganglion (VG). ChR2 is expressed in motor neurons using the GAL4-UAS system. B) Intracellular recording from a M6 muscle. 40 ms blue light pulses (at 127 µW / mm2) reliably evoke large synaptic potentials (asterisks) in M6.
Discussion
Critical steps involve both the initial dissection and the entering of muscle cells. If nerves are cut or muscle is damaged during the initial dissection it is difficult to continue the rest of the experiment. During dissection, one must be very careful to angle dissecting scissors upwards as much as possible during dorsal incision. During the second crucial step, entering a muscle cell, one must watch for a hyperpolarization past ~30 mV. Values above -30 mV indicate that the electrode is either not properly within a muscle cell or in an unhealthy cell.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Institutes of Health grants RO1GM-33205 and MH-067284 to L.C. Griffith and by a Brandeis University summer undergraduate research scholarship to N. J. Hornstein. Preliminary experiments for this technique were performed at the Marine Biological Laboratory as part of the 2008 Neural Systems and Behavior summer course (NIMH grant: R25 MH059472) in Woods Hole, MA.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
| Sylgard | Ellsworth adhesives | 4019862 | www.ellsworth.com | |
| Minutens Pins | Fine Science Tools | 26002-10 | www.finescience.com | |
| Dissecting Dish | Fisher Scientific | www.fishersci.com | ||
| Neuroprobe Intracellular Amplifier and Head Stage | A-M Systems | 680100 | www.a-msystems.com | |
| Powerlab 4/30 data acquisition system | AD instruments | www.adinstruments.com | ||
| Grass stimulator | Grass instruments | www.grasstechnologies.com | ||
| Desktop Computer | Dell | www.dell.com | ||
| Dissecting Scope | Leica | www.leica-microsystems.com | ||
| Light Source | Dolan-Jenner | 41446-062 | www.dolan-jenner.com | |
| Fly Media | ||||
| All-Trans-Retinal | Sigma-Aldrich | 116-31-4 | www.sigmaaldrich.com | |
| OK-371 Gal4 Flies | Aberle lab, Griffith lab, Bloomington stock center | |||
| UAS-ChR2 Flies | Fiala lab, Griffith lab | |||
| LED controller circuit | Built in Griffith lab | http://www.ledsupply.com http://www.futureelectronics.com Composed of: 1. 200 mA Buck Puck 2. Blue LED 3. Insulated wire 4. Circuit bread board |
||
| LED Heat Sink | Thor Labs | http://www.thorlabs.com/ | ||
| Air Table | TMC | http://www.techmfg.com/products/accessories/intro3.html | ||
| Faraday Cage | Built in Griffith lab | |||
| Leica Leitz M Micro-Manipulator | Leica Leitz | ACS01 | www.leica-microsystems.com | |
| Electrode Holder | Axon Instruments | www.axon.com | ||
| Borosilicate Glass | FHC | www.fh-co.com/p14-15.pdf | ||
| Electrode Puller | Sutter Instruments | www.sutter.com | ||
| HL 3.1 Saline with 0.8mM Ca2+ | Contents (mM): NaCl:70 KCl:5 CaCl2: 0.8 MgCl2:4Sucrose:115 NaHCO3: 10 Trehalose: 5 HEPES |
|||
| Micro-Dissection Tools | Fine Science Tools | www.finescience.com |
References
- Keshishian, H., Broadie, K., Chiba, A., Bate, M. The Drosophila neuromuscular junction: a model system for studying synaptic development and function. Annu Rev Neurosci. 19, 545 (1996).
- Collins, C. A., DiAntonio, A. Synaptic development: insights from Drosophila. Curr Opin Neurobiol. 17 (1), 35 (2007).
- Lagow, R. D., et al. Modification of a hydrophobic layer by a point mutation in syntaxin 1A regulates the rate of synaptic vesicle fusion. PLoS Biol. 5 (4), e72 (2007).
- Nagel, G., et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 15 (24), 2279 (2005).
- Nagel, G., et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 100 (24), 13940 (2003).
- Schroll, C., et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr Biol. 16 (17), 1741 (2006).
- Lin, D. M., Auld, V. J., Goodman, C. S. Targeted neuronal cell ablation in the Drosophila embryo: pathfinding by follower growth cones in the absence of pioneers. Neuron. 14 (4), 707 (1995).
- Ralph Greenspan, . . Fly Pushing: The Theory and Practice of Drosophila Genetics, 2nd ed. , (2004).

