Transsynaptic Tracing from Peripheral Targets with Pseudorabies Virus Followed by Cholera Toxin and Biotinylated Dextran Amines Double Labeling
Summary
Transsynaptic tracing has become a powerful tool for analyzing central efferents regulating peripheral targets through multi-synaptic circuits. Here we present a protocol that exploits the transsynaptic pseudorabies virus to identify and localize a functional brain circuit, followed by classical tract tracing techniques to validate specific connections in the circuit between identified groups of neurons.
Abstract
Transsynaptic tracing has become a powerful tool used to analyze central efferents that regulate peripheral targets through multi-synaptic circuits. This approach has been most extensively used in the brain by utilizing the swine pathogen pseudorabies virus (PRV)1. PRV does not infect great apes, including humans, so it is most commonly used in studies on small mammals, especially rodents. The pseudorabies strain PRV152 expresses the enhanced green fluorescent protein (eGFP) reporter gene and only crosses functional synapses retrogradely through the hierarchical sequence of synaptic connections away from the infection site2,3. Other PRV strains have distinct microbiological properties and may be transported in both directions (PRV-Becker and PRV-Kaplan)4,5 . This protocol will deal exclusively with PRV152. By delivering the virus at a peripheral site, such as muscle, it is possible to limit the entry of the virus into the brain through a specific set of neurons. The resulting pattern of eGFP signal throughout the brain then resolves the neurons that are connected to the initially infected cells. As the distributed nature of transsynaptic tracing with pseudorabies virus makes interpreting specific connections within an identified network difficult, we present a sensitive and reliable method employing biotinylated dextran amines (BDA) and cholera toxin subunit b (CTb) for confirming the connections between cells identified using PRV152. Immunochemical detection of BDA and CTb with peroxidase and DAB (3, 3'-diaminobenzidine) was chosen because they are effective at revealing cellular processes including distal dendrites6-11.
Introduction
Transsynaptic tracing has become a powerful tool used to analyze central efferents that regulate peripheral targets through multi-synaptic circuits. This approach has been most extensively used in the rodent brain by utilizing the swine pathogen pseudorabies virus (PRV), especially the attenuated strain PRV-Bartha first described in 196112. Here, we present a protocol for identifying the motor cortical representation of specific muscles or muscle groups using a recombinant pseudorabies virus strain (PRV152) expressing the enhanced green fluorescent protein (eGFP) reporter gene2. The described method exploits the behavior of neurotropic viruses, which produce infectious progeny that cross synapses to infect other neurons within a functional circuit3,4,13. PRV152, which is isogenic with PRV-Bartha, only crosses synapses retrogradely through the hierarchical sequence of synaptic connections away from the infection site3,5. By precisely controlling the peripheral site of infection it is possible to limit the entry of the virus into the brain through a specific subset of motor neurons. As the virus sequentially infects chains of connected neurons, the resulting pattern of eGFP signal throughout the brain will then resolve the network of neurons that are connected to the initially infected cells.
An additional advantage of using virus for neural tracing is the amplification of the reporter protein (eGFP in this case) within infected cells. This signal amplification provides a level of sensitivity that allows detection of even sparse projections. For example, a sparse projection from vibrissa motor cortex to the facial motor neurons controlling the whiskers was found in rats using virally expressed green fluorescent protein14; previous studies failed to find this projection using classical tracers without reporter gene amplification11,15. Unfortunately, many viral tracing vectors, like the one used in the cited study, do not cross synapses, thereby limiting their use for tracing multi-synaptic circuits.
While presenting distinct advantages for identifying the network of cells participating in a motor circuit, the distributed nature of transsynaptic tracing with PRV-152 makes interpreting specific connections within the circuit difficult. Therefore, we present a simple method for validating specific connections within circuits identified using PRV-152 by double-labeling using biotinylated dextran amines (BDA) and cholera toxin subunit b (CTb). The combined use of BDA and CTb is a well-established approach for tracing connections between specific sets of neurons6-8,11. When used together, these two tracers can be visualized in the same section using a two-color DAB (3, 3'-diaminobenzidine) procedure16. High molecular weight BDA (BDA10kDa) was selected for this protocol because it yields detailed labeling of neuronal processes6,7,9. Additional advantages of BDA10kDa include the following: it is preferentially transported in the anterograde direction6-8; it can be delivered by iontophoretic or pressure injection6-8; it can be visualized by a simple avidin-biotinylated HRP (ABC) procedure17; and it can be imaged by light or electron microscopy6,7,18. Immunochemical detection of CTb with peroxidase and DAB was chosen for retrograde labeling of motoneurons because it is effective at revealing cellular processes including distal dendrites10,19. We recently used this approach to identify the vocal motor pathway in mice and to reveal a sparse connection from primary motor cortex to the laryngeal motor neurons, which was previously assumed to be absent20.
Protocol
NOTE: All animal procedures have been reviewed and approved by the Duke University Institutional Animal Care & Use Committee.
1. Storing Pseudorabies Virus
- We obtain live virus (PRV152) from the laboratory of Dr. Lynn Enquist at Princeton University at a titer of 1 x 109 pfu/m. The protocol to generate the virus has been published2.
- Aliquot the virus at 20 µl per tube inside a BSL-2 biosafety cabinet and store at -80 °C under appropriate biosafety conditions.
- Thaw an aliquot of PRV immediately before injecting.
2. Surgical Preparation for Injections into Muscle
- Induce general anesthesia by intramuscular injection of ketamine-xylazine (100 mg/kg ketamine; 10 mg/kg xylazine) and maintain an appropriate anesthetic plane using isofluorane.
- Protect the corneas with an opthalmic ointment.
- Prepare the surgical site according to aseptic technique by trimming hair and disinfecting the surgical site with alternating scrubs of Betadine and 70% alcohol (minimum of 3 cycles). Be sure to use sterile drapes to cover surgical areas. Make sure to adhere to sterile techniques throughout the procedures.
- Make a skin incision and reveal the muscle of interest. For example, to access the cricothyroid laryngeal muscle it is necessary to first remove the overlying portion of the sternohyoid muscle.
- Seal any transected muscles with VetBond tissue adhesive.
3. Injection of PRV into Muscle
- Load the 10 µl NanoFil microsyringe system with freshly thawed PRV solution, attach a 34 G stainless steel needle, and carefully mount it on the stereotaxic device.
- Slowly clear the dead space and verify that solution exits the microsyringe tip. Discard fluid as bioharzard waste.
- Using a stereotaxic micropositioning device carefully place the microsyringe tip into the muscle of interest and slowly fill the muscle until slight swelling is visible. The injection rate will vary depending on the size of the muscle and volume to be injected. For example, five injections of 200 nl (1 µl total) at a rate of 4 nl/sec should be made 1 min apart at the same site to fill the cricothyroid muscle. Move the syringe to the next muscle of interest (the lateral cricoarytenoid in this case) and repeat the injection procedure. Only puncture each muscle once.
- Retract the microsyringe after five minutes.
- Seal the break in the fascia using VetBond tissue adhesive.
- After all injections have been completed, close the wound using VetBond tissue adhesive. Depending on your local institional animal use guidelines, sutures or wound clips may be used.
- Monitor the animal until sternal recumbency and provide analgesia, food, water, and care as required by your institutional animal use guidelines.
- After the experimentally determined survival time (in this case, 90 hr to label 2nd order cortical neurons), sacrifice the animal by pentobarbital overdose and perfuse transcardially with 0.9% saline followed by 4% formaldehyde in 0.1 M PBS.
- Remove and post-fix the brain in 4% formaldehyde for 24 hr.
- Cryoprotect the brain in phosphate buffer containing 30% sucrose for at least 48 hr.
4. Immunochemical Detection of eGFP
- Cut sections at 40 µm on a sliding microtome and save floating sections into 0.1M PBS. Thinner sections, for example 30 µm, may be used when staining mounted sections.
- Quench sections for 30 min in 0.3% H2O2 in PBS protected from light.
- Block non-specific antigens in the sections for 30 min in PBS containing 0.3% Tween 20 with normal goat serum from the VECTASTAIN Elite Kit (VE kit).
- Incubate blocked sections for 3.5 hr in rabbit anti-eGFP (1:1,000) at RT.
- Wash sections three times in PBS for 5 min, then incubate them for 1 hr in goat anti-rabbit secondary antibody from the VE kit at RT.
- Prepare the ABC solution from the VE kit according to the manufacturer’s instructions.
- Wash sections three times in PBS for 5 min, then react them for 1 hr in ABC solution from the VE kit at RT.
- Wash sections three times in phosphate buffer for 10 min, and develop for 8 min in phosphate buffer, pH 7.4, containing 0.05% DAB (3, 3'-diaminobenzidine) and 0.015% H2O2.
- Mount sections on SuperFrost Plus slides and dehydrate them through a graded alcohol series (70%, 95%, 100% and 100% for 5 min each).
- Clear the sections through two xylene washes (5 min each) and coverslip the slides with Permount mounting medium.
- Image the mounted sections on a microscope. The DAB reaction product inside the cells should appear brown. Digitized images can be color inverted to highlight fine processes.
5. Surgical Preparation for Injection of Tracers into Brain Regions Discovered by PRV Tracing
- Induce general anesthesia by intramuscular injection of ketamine-xylazine (100 mg/kg ketamine; 10 mg/kg xylazine) and maintain an appropriate anesthetic plane using isofluorane.
- Protect the corneas with an opthalmic ointment.
- Fix the head in an appropriate stereotaxic frame.
- Prepare the surgical site according to aseptic technique by trimming hair and disinfecting the site with alternating scrubs of Betadine and 70% alcohol (minimum of 3 cycles).
- Make a scalp incision and retract the skin over the brain region of interest.
- Perform a small craniotomy at the appropriate stereotaxic coordinates. For example, the coordinates for laryngeally connected motor cortex identified by PRV tracing in an adult mouse are 1.2 mm lateral and 0.2 mm rostral to Bregma.
6. Injection of Biotinylated Dextran Amines into Brain
- Prepare 7.5% biotinylated dextran amines (BDA) by dissolving 25 mg of BDA (10,000 MW) in 333 µl of sterile saline.
- Load the Nanoject II micropipette system with sufficient BDA solution for the planned injections.
- Slowly clear the dead space and verify that solution is exiting the micropipette tip.
- Using a stereotaxic micropositioning device carefully lower the micropipette tip into the brain region of interest and slowly inject BDA. Adjust the injection rate depending on the size of the region to be labeled. For example, 12 injections of 4.6 nl should made be at four different locations 0.2 mm apart (rostro-caudal direction) to cover the laryngeally connected motor cortex in adult mice. The final injection volume should be adjusted according to the size of the brain region of interest, keeping in mind that label may spread from the injection core by diffusion through brain tissue and along the processes of labeled neurons.
- Seal the craniotomy using dental cement, and close the scalp wound using VetBond tissue adhesive.
- Monitor the animal until sternal recumbency and provide analgesia, food, water, and care as required by your institutional animal use guidelines.
7. Injection of Cholera Toxin Subunit b into Muscle
- Prepare 1% Cholera Toxin subunit b (CTb) by dissolving 1 mg of CTb in 100 µl of sterile saline.
- Six days after BDA injection into the brain, perform the surgical preparation as described above for PRV injections into muscle.
- Load the 10 µl NanoFil microsyringe system with CTb solution, attach a 34 G stainless steel needle, and carefully mount it on the stereotaxic device.
- Perform the microinjections into the muscle(s) of interest as previously described for PRV.
- Monitor the animal until sternal recumbency and provide analgesia, food, water, and care as required by your institutional animal use guidelines.
- Three days after CTb injection, sacrifice the animal by pentobarbital overdose and perfuse transcardially with 0.9% saline followed by 4% formaldehyde in 0.1 M PBS.
- Remove and post-fix the brain in 4% formaldehyde for 24 hr.
- Cryoprotect the brain in phosphate buffer containing 30% sucrose for at least 48 hr.
8. Detection of BDA and CTb in the Same Sections
- Cut sections at 40 µm on a microtome and save floating sections into 0.1M PBS.
- Quench sections for 30 min in 0.3% H2O2 in PBS protected from light.
- Prepare the ABC solution from the VE kit according to the manufacturer’s instructions.
- Wash sections three times in PBS for 5 min, then react them for 1 hr in ABC solution at RT.
- Wash sections three times in phosphate buffer for 10 min, and develop for 8 min in phosphate buffer, pH 7.4, containing 0.05% DAB (3, 3'-diaminobenzidine), 0.015% H2O2, and 0.05% nickel chloride. The DAB reaction product inside the cells should appear black.
- Block sections for 30 min in PBS containing 0.3% Tween 20 with normal rabbit serum from the VECTASTAIN Elite Kit.
- Incubate blocked sections for 2 hr in goat anti-CTb (1:10,000) at RT.
- Wash sections three times in PBS for 5 min, then incubate them for 1 hr in rabbit anti-goat secondary antibody from the VE kit at RT.
- Prepare the ABC solution from the VE kit according to the manufacturer’s instructions.
- Wash sections three times in PBS for 5 min, then react them for 30 min in ABC solution at RT.
- Wash sections three times in phosphate buffer for 10 min, and develop for up to 8 min in phosphate buffer, pH 7.4, containing 0.05% DAB (3, 3'-diaminobenzidine) and 0.015% H2O2. The DAB reaction product inside the cells should appear brown.
- Mount sections on SuperFrost Plus slides and dehydrate them through a graded alcohol series (70%, 95%, 100% and 100% for 5 min each).
- Clear the sections through two xylene washes (5 min each) and coverslip the slides with Permount mounting medium.
- Image the mounted sections on a microscope.
Representative Results
Staining for eGFP should begin showing weak signal in primary motor neurons approximately 72 hr after injecting PRV152 into muscle. The replication and transsynaptic transport of virus are titer- and time-dependent4. Approximately 90 hr after injection, eGFP staining will reveal robust signal in 2nd order infected cells. Longer survival times will reveal 3rd and higher order cells but survival times are limited by the lethality of PRV at approximately 5 days after inoculation.
Figure 1a shows neurons infected with PRV152 expressing eGFP in nucleus ambiguus, which houses the laryngeal motor neurons, 94 hr after injection of PRV152 into two of the seven laryngeal muscles. Because the virus entered the brain through neurons that innervate the infected muscles, other phonatory motor neuron pools, such as the hypoglossal nucleus, do not express eGFP (Figure 1b).
Figure 2 shows retrograde transport of the virus to 2nd order brainstem neurons subsequent to primary infection in nucleus ambiguus. The injection was unilateral, and the resulting pattern of label in the brainstem shows infection of the ipsilateral nucleus ambiguus and solitary nucleus (Figure 2a). Reticular interneurons were also infected and stained strongly for eGFP (Figure 2b). These interneurons connect various structures, including the phonatory motor neuron pools and the contralateral nucleus ambiguus; however, their efferent targets remain unlabeled because the virus only spreads in the retrograde direction.
Figure 3 shows infected premotor neurons in the motor cortex expressing eGFP contralateral to the peripheral injection site. Because PRV152 spreads through multi-synaptic circuits impinging on laryngeal motor neurons in nucleus ambiguus, it can be assumed that only the population of cells that compose the motor cortical representation of the laryngeal musculature were infected. The lack of eGFP signal in the rest of the section shown (and most of the forebrain not shown) demonstrates the specificity of the labeling.
Figure 4 shows BDA-labeled axons in the brainstem at the level of nucleus ambiguus. The axons make contact with the CTb-positive motor neurons retrogradely labeled by injections into laryngeal muscles. Axons are shown forming varicosities and putative terminal boutons near the dendrites and soma of laryngeal motor neurons. Further experiments using electron microscopy or electrophysiology are required to determine whether these varicosities represent synaptic boutons.
Figure 5a shows a bilateral injection of BDA placed into the laryngeally connected motor cortex as identified by retrograde transynaptic tracing with PRV152. Because BDA is not directionally specific, both afferent and efferent connections may be labeled. For example, both a projection field (anterograde) and cell bodies (retrograde) label are seen in the thalamus (Figure 5b).
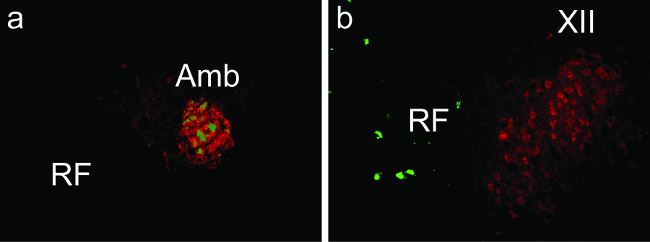
Figure 1: Neurons Infected with PRV152 Expressing eGFP (green) and Choline Acetyltransferase Immunopositive Motoneurons (red) in the (a) Ambiguus (Amb) and (b) Hypoglossal (XII) Nuclei of the Mouse Brainstem 94 hr after Injection of PRV152 into the Cricothyroid and Lateral Cricoarytenoid Laryngeal Muscles. RF = reticular formation. Please click here to view a larger version of this figure.
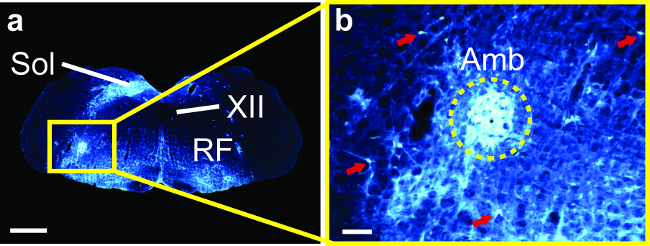
Figure 2: Psuedorabies Virus (PRV152) Infection in the Brainstem of a Mouse 86 hr after Injecting PRV152 into the Cricothyroid and Lateral Cricoarytenoid Laryngeal Muscles. (a) Neurons expressing enhanced green fluorescent protein (white; colors inverted from original brightfield images of stained sections) in nucleus ambiguus (Amb) ipsilateral to the injected muscle, the surrounding reticular formation (RF) and the solitary nucleus (Sol), but not the hypoglossal nucleus (XII). (b) Amb and reticular interneurons at higher magnification (red arrows, cell bodies in RF). Scale bars: 1 mm for a; 100 µm for b. (Modified from Arriaga et al. 2012). Please click here to view a larger version of this figure.
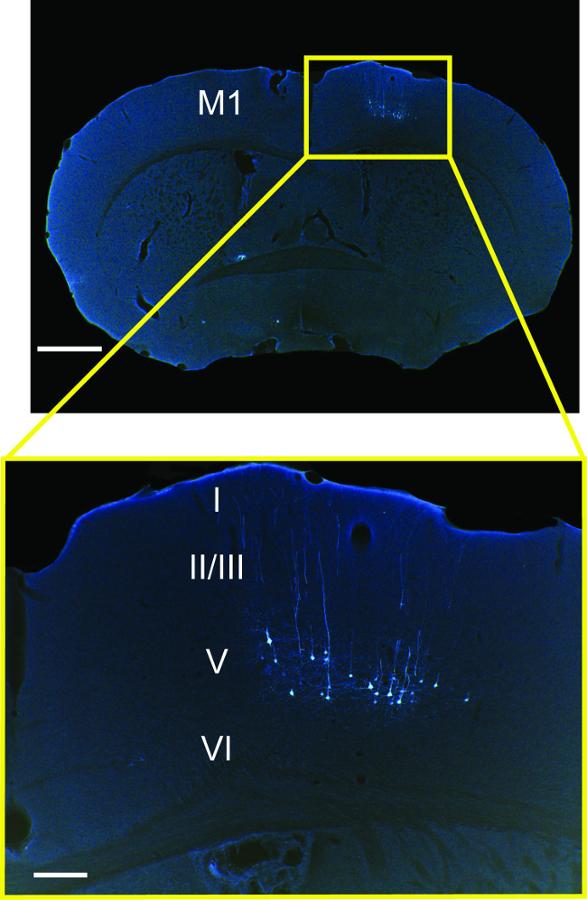
Figure 3: Cortical Pyramidal Neurons Expressing eGFP (white; colors inverted from original brightfield images of stained sections) in Cortical Layer V of M1 following Injection of Psuedorabies Virus (PRV152) into the Cricothyroid and Lateral Cricoarytenoid Laryngeal Muscles. Scale bars: 1 mm for main panel; 200 µm for inset. (Modified from Arriaga et al. 2012).
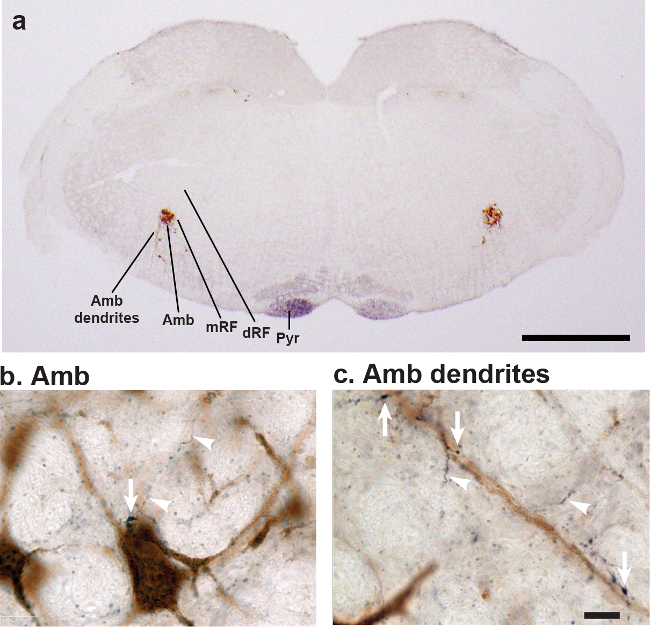
Figure 4: M1 Axons in the Brainstem. (a) Low power of a coronal brainstem section containing CTb-labeled motor neurons in Amb (brown) from an injection in laryngeal muscles and M1 axons (black) from an injection of BDA into M1. BDA labelled axons can be seen in the cortico-pyramidal (Pyr) track. Abbreviations: Amb, nucleus ambiguus; Pyr, pyramids; mRF, reticular formation directly medial to Amb; dRF, reticular formation dorsal to Amb. (b) High magnification of BDA labeled axon (black, arrow heads) from M1 that makes contact (arrow) onto a Amb motor neurons (CTb brown). (c) M1 axons (black) running along (arrows) and near (arrow heads) a large Amb motor neuron dendrite that radiates out from Amb. Please click here to view a larger version of this figure.
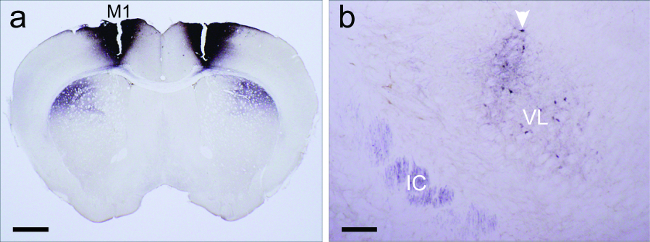
Figure 5: Injection site and additional bidirectional connections revealed by BDA injections not seen with PRV transynaptic tracing. (a) Bilateral biotinylated dextran amine (BDA) injections (black) in primary motor cortex (M1) revealed a dense terminal projection field (black) in the underlying striatum. (b) Cortical axons from M1 terminate in the ventral lateral nucleus of the thalamus (VL); A cluster of thalamic cells (dark brown and marked by white arrow head) that project back to the M1 singing region is also observed in VL. The internal capsule (IC) has labeled axons coming from the cortex. Scale bars: 1 mm for a; 200 µm for b. (Modified from Arriaga et al. 2012). Please click here to view a larger version of this figure.
Discussion
There are a number of issues that must be taken into consideration when planning an experiment using PRV1524,21. Most importantly, pseudorabies virus is lethal. As mentioned previously, great apes, including humans are not susceptible to infection, but appropriate care must be exercised to protect other animals. Adult mice typically survive five to seven days after inoculation with the attenuated PRV152 strain. Therefore, PRV152 is not appropriate for experiments that require survival times longer than one week. Moreover, infected animals typically display signs of illness during the survival period, thereby limiting the utility of PRV152 for experiments requiring observation of normal behavior.
Additionally, PRV152 triggers a cytotoxic immune response22. Because the replication and transmission of virus are titer- and time-dependent4, the pattern of neurons revealed by immunochemical detection of viral proteins or the eGFP reporter gene will change over the course of the survival period as cells progress through stages of infection. Five days after inoculation in muscle, 3rd or 4th order neurons may be detectable, but the earliest infected neurons may become apoptotic. Thus the optimal survival time should be determined experimentally for each study. It is recommended to perform a time-series analysis to determine the progression of infection through a circuit and the best time point for labeling the specific neurons of interest without risking cell or animal death.
One of the most important factors affecting the success of labeling with PRV is the viral titer. Marked reductions in the number of infected cells have been reported when decreasing the titer of PRV-Bartha from 1.4 x 105 to 7 x 104 pfu/ml23. Therefore, we recommend using titers above 1 x 107 pfu/ml and keeping aliquoted virus at -80°C until it is used to maintain its infectivity. Even when using a high titer, one should keep in mind that not all cell types may be permissive for infection. Therefore, the cells identified by PRV tracing may not be completely representative of the inputs to an infected target.
The described method focuses on the utility of PRV for analyzing central efferents regulating peripheral targets, specifically muscles, through multi-synaptic circuits. Other neural circuits, such as visual and autonomic24, are well suited for this technique because they can also be infected by peripheral inoculation. However, PRV can be used effectively for direct injection into the central nervous system when some issues are carefully considered, as discussed extensively in a prior review3. Those authors mention that PRV-Bartha and related strains extensively fill the dendritic arbors of infected neurons, making PRV152 well suited for identifying afferents to distal dendrites. This property of the virus makes the described technique particularly useful for deducing a preliminary list of inputs to distal dendrites located outside of the cell body region that is typically studied with conventional tracers.
When tracing from peripheral targets, sympathetic innervation should be considered a possible confound. Therefore, we recommend initially comparing labeling patterns in normal and sympathectomized animals to determine whether PRV labeling of sympathetic inputs will affect interpretation of the results.
Signal in infected cells from eGFP expressed by PRV152 can be imaged directly using fluorescence microscopy without the need for immunohistochemistry; however, this signal is subject to photo-bleaching and degrades over time. The main advantage of immunohistochemistry combined with DAB staining for tract tracing is the permanent nature of the reaction products. While it is also possible to use antibodies against viral proteins to detect infected cells, different viral elements will not necessarily co-localize with each other25 or with the eGFP signal. Therefore, we recommend using antibodies against eGFP to preserve the labeling pattern observed by fluorescence.
Because all afferents to an infected cell are likely to become conduits for transsynaptic passage of virus, including those that contact distal dendrites, we considered it important to select a conventional tracer that reliably stains such dendrites for subsequent confirmation of relays in the identified circuit. We selected CTb for this purpose, and found that it provides good staining of the dendrites and strong contrast with both the surrounding neuropil and the descending axons from the motor cortex. Although it is possible to reverse the coloration of the stained axons and cell bodies (BDA = brown; CTb = black), we find that black provides the best contrast for visualizing fine caliber axons.
An important point to keep in mind when interpreting BDA labeling patterns is that BDA10kDa is mainly used as an anterograde tracer but can also label cells retrogradely (Figure 6)6. Moreover, BDA can be transported retrogradely into a cell then distributed along an axon collateral to another terminal site. If those axon collaterals are present in the same region as the projection target of the injected region, then it is difficult to distinguish them from anterograde labeling.
A final technical note is that the 34 G NanoFil metal syringe performs better than a fine caliber, pulled glass pipette for injecting tracers into muscles, but causes too much mechanical damage when used for intracerebral injections in small animals. We therefore recommend using the metal syringe for peripheral injections and the glass pipette for central injections.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Toshio Terashima of Kobe University, Japan, for teaching the laryngeal surgery technique, and Dr. Lynn Enquist of Princeton University for supplying PRV-Bartha. Research was supported by NIH pioneer award DP1 OD000448 to Erich D. Jarvis and an NSF Graduate Research Fellowship award to Gustavo Arriaga. Figures from appropriately credited previous work are used under the PLoS ONE open access Creative Commons license (CC-BY) in accordance with the journal’s editorial policies.
Materials
| Name of Reagent/Material | Company | Catalog Number | Comments |
| NanoFil Microinjection System | World Precision Instruments | IO-Kit | 34 gauge option |
| Stereotaxic frame | David Kopf Instruments | Model 900 | |
| Nanoject II Auto-Nanoliter Injector | Drummond Scientific Company | 3-000-204 | |
| Sliding microtome | Leica | SM2010 R | |
| [header] | |||
| VetBond | 3M | 1469SB | |
| Isofluorane (Forane) | Baxter | 1001936060 | |
| Betadine Swab Stick | Cardinal Health | 2130-01 | 200 count |
| Permount Mounting Medium | Fisher Scientific | SP15-500 | |
| SuperFrost Plus slides | Fisher Scientific | 12-550-15 | |
| Biotinylated dextran amines | Invitrogen | D-1956 | 10,000 MW |
| Pseudorabies virus | Laboratory of Dr. Lynn Enquist (Princeton University) | PRV152 | Titer > 1 x 107 |
| Anti-Cholera Toxin B Subunit (Goat) | List Biological Laboratories | 703 | |
| Cholera Toxin B Subunit | List Biological Laboratories | 103B | |
| Anti-eGFP | Open Biosystems | ABS4528 | |
| 3, 3'-diaminobenzidine | Sigma-Aldrich | D5905 | 10 mg tablets |
| Ethanol | Sigma-Aldrich | E7023 | 200 proof |
| Formaldehyde | Sigma-Aldrich | F8775 | Dilute to 4% |
| Hydrogen peroxide | Sigma-Aldrich | H3410 | 30% |
| Ketamine HCl & Xylazine HCl | Sigma-Aldrich | K4138 | 80 mg/mL & 6 mg/mL |
| Nickel chloride | Sigma-Aldrich | 339350 | |
| Phosphate buffer | Sigma-Aldrich | P3619 | 1.0 M; pH 7.4 |
| Phosphate buffered saline | Sigma-Aldrich | P5493 | 10X; pH 7.4 |
| Sodium Pentobarbital | Sigma-Aldrich | P3761 | 50 mg/mL dose |
| Sucrose | Sigma-Aldrich | S9378 | |
| Tween 20 | Sigma-Aldrich | P1379 | |
| Xylenes | Sigma-Aldrich | 534056 | Histological grade |
| VECTASTAIN Elite ABC Kit | Vector Laboratories | PK-6101 (rabbit); PK-6105 (goat) | |
| Optixcare opthalmic ointment | Vet Depot | 1017992 |
References
- Card, J. P., Enquist, L. W. . Transneuronal circuit analysis with pseudorabies viruses.Multiple values selected. Unit 1.5, 1.51-1.5.28 (2001).
- Smith, B. N., Banfield, B. W., et al. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proceedings of the National Academy of Sciences of the United States of America. 97 (16), 9264-9269 (2000).
- Aston Jones, G., Card, J. P. Use of pseudorabies virus to delineate multisynaptic circuits in brain opportunities and limitations. Journal of Neuroscience Methods. 103 (1), 51-61 (2000).
- Pomeranz, L. E., Reynolds, A. E., Hengartner, C. J. Molecular biology of pseudorabies virus impact on neurovirology and veterinary medicine. Microbiology and Molecular Biology Reviews. 69 (3), 462-500 (2005).
- Brittle, E. E., Reynolds, A. E., Enquist, L. W. Two modes of pseudorabies virus neuroinvasion and lethality in mice. Journal of Virology. 78 (23), 12951-12963 (2004).
- Reiner, A., Veenman, C. L., Medina, L., Jiao, Y. Pathway tracing using biotinylated dextran amines. Journal of neuroscience. 103, 23-37 (2000).
- Reiner, A., Honig, M. G. Neuroanatomical tract-tracing 3 (Chapter 10). Dextran Amines Versatile Tools for Anterograde and Retrograde Studies of Nervous System Connectivity. 10, 304-335 (2006).
- Veenman, C. L., Reiner, A., Honig, M. G. Biotinylated dextran amine as an anterograde tracer for single and double labeling studies. Journal of Neuroscience Methods. 41 (3), 239-254 (1992).
- Rajakumar, N., Elisevich, K., Flumerfelt, B. A. Biotinylated dextran a versatile anterograde and retrograde neuronal tracer. Brain Research. 607 (1-2), 47-53 (1993).
- Dederen, P. J. W. C., Gribnau, A. A. M., Curfs, M. H. J. M. Retrograde neuronal tracing with cholera toxin B subunit: comparison of three different visualization methods. Histochemical Journal. 26 (11), 856-862 (1994).
- Hattox, A. M., Priest, C. A., Keller, A. Functional circuitry involved in the regulation of whisker movements. The Journal of Comparative Neurology. 442 (3), 266-276 (2002).
- Bartha, A. Experimental reduction of virulence of Aujeszkys disease virus. Magy Allatorv Lapja. 16, 42-45 (1961).
- Kuypers, H., Ugolini, G. Viruses as transneuronal tracers. Trends in Neurosciences. 13 (2), 71-75 (1990).
- Grinevich, V., Brecht, M., Osten, P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. The Journal of Neuroscience. 25 (36), 8250-8258 (2005).
- Miyashita, E., Keller, A., Asanuma, H. Input output organization of the rat vibrissal motor cortex. Experimental Brain Research. 99 (2), 223-232 (1994).
- Hsu, S. M., Soban, E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. Journal of Histochemistry and Cytochemistry. 30 (10), 1079-1082 (1982).
- Hsu, S. M., Raine, L., Fanger, H. Use of avidin biotin-peroxidase complex (ABC) in immunoperoxidase techniques a comparison between ABC and unlabeled antibody (PAP) procedures. Journal of Histochemistry and Cytochemistry. 29 (4), 577-580 (1981).
- Wouterlood, F. G., Jorritsma Byham, B. The anterograde neuroanatomical tracer biotinylated dextran amine comparison with the tracer Phaseolus vulgaris leucoagglutinin in preparations for electron microscopy. Journal of Neuroscience Methods. 48 (1-2), 75-87 (1993).
- Altschuler, S. M., Bao, X. M., Miselis, R. R. Dendritic architecture of nucleus ambiguus motoneurons projecting to the upper alimentary tract in the rat. The Journal of Comparative Neurology. 309 (3), 402-414 (1991).
- Arriaga, G., Zhou, E. P., Jarvis, E. D. Of mice birds and men the mouse ultrasonic song system has some features similar to humans and songlearning birds. PLoS ONE. 7 (10), e46610 (2012).
- Card, J. P. Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neuroscience and Biobehavioral Reviews. 22 (6), 685-694 (1998).
- Zuckerman, F. A., Zsak, L., Mettenleiter, T. C., Ben Porat, T. Pseudorabies virus glycoprotein gIII is a major target antigen for murine and swine virus-specific cytotoxic T lymphocytes. Journal of Virology. 64 (2), 802-812 (1990).
- Card, J. P., Enquist, L. W., Moore, R. Y. Neuroinvasiveness of pseudorabies virus injected intracerebrally is dependent on viral concentration and terminal field density. The Journal of Comparative Neurology. 407 (3), 438-452 (1999).
- Pickard, G. E., Smeraski, C. A., et al. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. The Journal of Neuroscience. 22 (7), 2701-2710 (2002).
- Smith, G. A., Gross, S. P., Enquist, L. W. Herpesviruses use bidirectional fast axonal transport to spread in sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 98 (6), 3466-3470 (2001).

