Grafting of Beads into Developing Chicken Embryo Limbs to Identify Signal Transduction Pathways Affecting Gene Expression
Summary
By grafting beads soaked in growth factors or specific inhibitors of signaling pathways into developing embryos it is possible to directly test their effects in vivo. In this protocol beads are grafted into the limb bud to determine the effects of these molecules on gene expression and signal transduction.
Abstract
Using chicken embryos it is possible to test directly the effects of either growth factors or specific inhibitors of signaling pathways on gene expression and activation of signal transduction pathways. This technique allows the delivery of signaling molecules at precisely defined developmental stages for specific times. After this embryos can be harvested and gene expression examined, for example by in situ hybridization, or activation of signal transduction pathways observed with immunostaining.
In this video heparin beads soaked in FGF18 or AG 1-X2 beads soaked in U0126, a MEK inhibitor, are grafted into the limb bud in ovo. This shows that FGF18 induces expression of MyoD and ERK phosphorylation and both endogenous and FGF18 induced MyoD expression is inhibited by U0126. Beads soaked in a retinoic acid antagonist can potentiate premature MyoD induction by FGF18.
This approach can be used with a wide range of different growth factors and inhibitors and is easily adapted to other tissues in the developing embryo.
Introduction
Avian embryos have provided a powerful tool for the study of development for many years1. One of their most useful characteristics is that they are relatively easy to manipulate. External development makes it possible to open the egg to access the embryo and perform various micromanipulations including examples such as the classic quail-chick chimera system for studying cell fate2,3, the injection of retroviruses for overexpression in specific tissues during development4,5 and explant culture to identify developmental signaling sources6. More recently chimeras generated between unlabeled hosts with grafts from a transgenic chicken line expressing GFP has shown that the combination of classical grafting and genetic modification can provide important insights into development7,8.
The ease with which the avian embryo can be manipulated has made it an excellent model for studying limb development9. Application of specific growth factors to developing limbs in vivo has been instrumental in identifying factors that alter limb patterning10,11 and continues to provide insights into this process12. This approach has also been used to study the factors that regulate muscle development and has uncovered roles for numerous signals such as Wnts13, BMPs14 and HGF15.
Recently this technique has been used to investigate the signals controlling myogenic gene expression in the limb bud and has shown that interactions between FGF18 and retinoic acid can control the timing of MyoD expression16. Using a combination of growth factors and small molecules that can be loaded onto beads and then grafted directly into specific tissues at defined developmental stages gives the opportunity to intervene at almost any time and region during development. This has been used to investigate many processes including somite patterning17,18, neural specification19, neural crest migration20 and axis extension21.
Here we describe a method for grafting beads soaked in either growth factors or inhibitors into developing chicken limbs. This has been used to determine the effects of these signals on myogenesis by analyzing muscle specific gene expression with in situ hybridization. We describe grafts using either heparin soaked beads, which are used for growth factors, or AG 1-X2 beads for small hydrophobic molecules such as retinoic acid or small molecule inhibitors of specific signaling pathways. However other beads are also available which have been used to deliver both FGFs22 and Shh23.
Protocol
Ethics Statement: All these experiments follow the animal care and ethical guidelines of the University of Nottingham.
1. Preparation of Heparin Beads for Grafting
- Wash heparin beads thoroughly in PBS before use. Note: Beads can be stored at 4 °C as a slurry in PBS.
- Select beads for grafting by removing them from the stock with a 20 µl pipette into a 1 ml drop of PBS. Then use a micropipette set to 2 µl to transfer beads into a 20 µl drop of PBS in a 3 cm petri dish. Choose beads based on size and, using a stereo dissecting microscope, transfer selected beads with a P2 pipette tip; those around 100 µm diameter are ideal.
- Remove the PBS from the beads. Remove most of the liquid with a 20 µl pipette and the residual liquid by capillary action with fine watchmakers forceps. Note: It is important to remove as much as possible so the growth factor is not diluted when it is added to the beads.
- Pipette the growth factor directly onto the beads. For FGF18 add 0.5 µl of recombinant FGF18 reconstituted at 0.5 mg/ml in 0.1% BSA in PBS. Place several drops of water around the edges of the dish to prevent the liquid from evaporating.
- Incubate the beads for 1 hr at RT. Remove the drops of water from the dish with a Pasteur pipette and place on ice ready for grafting.
- If required (for transparent beads) transfer 4-5 beads to 2% phenol red before grafting to help visualize them. Rinse in PBS before transferring to the embryo.
2. Preparation of AG 1-X2 Beads for Grafting
- Prior to use derivatize beads with 0.2 N formic acid.
- To do this use a spatula to transfer beads to a 1.5 ml microcentrifuge tube. Add 1ml of 0.2 N formic acid and wash on a shaking platform for 1 hr.
- Then remove the formic acid with a p1000 pipette and replace with 1 ml of water. Wash for 1 hr and repeat six times to remove remaining formic acid.
- Remove remaining liquid with a p1000 pipette and store the beads at 4 °C. Note: It is not necessary to remove all the residual water at this stage.
- Remove a small pellet of beads of approximately 5-10 µl volume from the stock using a small spatula into a 3 cm petri dish and add 1 ml of DMSO (or whichever solvent the drug is dissolved in). Note: This should provide several hundred beads from which to select those of the right size.
- Then use a P2 pipette set to 2 µl to transfer beads of approximately 100 µm diameter to a 20 µl drop of solvent in another 3 cm petri dish.
- Remove solvent with a 20 µl pipette and any residual solvent with a 2 µl pipette. Replace with 20 µl of the drug to be applied.
NOTE: For consistency dissolve the drug at a concentration of 100 µM in DMSO (or which ever solvent is used), aliquot into 20 µl and store at -80 °C as some small molecule inhibitors are unstable and it is best to avoid repeated freeze-thawing. If necessary then dilute the drug to the desired concentration before soaking beads. Effective concentrations may need to be determined empirically for each drug. - Incubate for 1 hr. Protect from light as many of these molecules are light sensitive.
- Before grafting transfer 4-5 beads with a p2 pipette set to 2 µl into 20 µl of 2% phenol red dissolved in water. Perform this step using a stereo dissecting microscope to visualize the beads. Remove a single bead with fine watchmakers forceps or a wire loop and rinse in PBS before transferring to the embryo. Do not leave beads in 2% phenol red for more than 15 min before use as this can cause loss of activity.
3. Preparing Tungsten Needles
- Cut fine tungsten wire into 3-4 cm lengths. Insert one piece of wire into the end of a glass Pasteur pipette and melt in a Bunsen burner to fix in position. Prepare up to 10 needles to ensure that there are spares if they are damaged during use.
- Place the end of the wire into a blowtorch flame and hold it there until the tungsten glows white and the tip is sharp (around 2-3 min). Note: During use the needle can be cleaned and re-sharpened in the blowtorch as needed.
NOTE: These needles can also be used to make loops for transferring beads. Gently touch the needle to the bench and it will bend to form a loop.
4. Preparing Embryos for Bead Grafts
- Incubate eggs with the blunt end up until they reach the desired stage (3-5 days for most limb bud manipulations). Using blunt forceps tap on the blunt end to break the shell and then use the forceps to remove the shell and expose the embryo. Ensure the eggshell membrane is also removed.
NOTE: 1-2 ml of PBS can be added with a Pasteur pipette to assist with this if the membrane sticks to the yolk. Gently pipette the PBS onto the surface of the yolk, grasp the membrane with the forceps, taking care not to damage the yolk, and gently pull it away from the egg. - Remove up to 5 ml of albumen from the egg with a 10 ml syringe and a 19 G needle. Take only as much as needed to ensure the embryo does not make contact with the tape used to seal the egg as removal of larger volumes can reduce embryo survival rates.
- To visualize the embryo add a 5-6 drops of artists india ink to 15 ml of PBS containing 100 U penicillin and 0.1 mg streptomycin/ml. Inject 0.5-1 ml directly under the embryo with a 1 ml syringe and a 25 G needle.
- Add 2-3 ml of PBS with 1% fetal calf serum and 100 U penicillin and 0.1 mg streptomycin/ml into the egg to ensure the embryo does not dehydrate. Using sharpened watchmakers forceps remove the vitelline membrane and open the amnion over the developing limb buds. Ensure the embryo does not dry out and, if needed, add more PBS / FCS.
NOTE: The limb buds can be clearly seen as paired outgrowths on the flank of the embryo. At these stages the embryo will turn such the right side is normally uppermost. As a result it is normally easier to graft beads into the right limbs and use the left ones as contralateral controls. - Use a sharpened tungsten wire to make an incision into the limb bud where the bead will be implanted. Avoid cutting all the way through the limb bud where possible although at younger stages this is difficult.
NOTE: In older embryos (HH stage 22 and above) it is possible to select specific regions of the limb bud for grafting but at younger stages this is more difficult as the limb bud itself is small. It is also important to remember that the limb bud will grow past the grafted bead so, especially at younger stages, the bead will remain in the proximal limb. - Pick up a bead from either the growth factor or drug using either extra fine watchmakers forceps or a loop made from a tungsten needle. If the bead has been soaked in either DMSO or phenol red, rinse in PBS before applying to the embryo. Transfer the bead to the embryo with the forceps / wire loop. Then use the sharpened tungsten wire to insert the bead into the incision.
- Add 1-2 ml of PBS/FCS to the egg ensuring that the embryo is kept hydrated but avoid applying directly onto the embryo itself as this can damage the embryos and cause the bead to be dislodged. Seal the egg with tape and return to the incubator.
Representative Results
At HH stage 21 MyoD is not expressed in developing limb myoblasts although staining can be seen in the myotome of the developing somites (Figure 1A). Figure 1B shows in situ hybridization for MyoD 6 hr following an FGF18 bead graft. MyoD is induced in myoblasts close to the bead while there is no expression in the contralateral limb. Co-grafting a bead soaked in U0126, a specific inhibitor of MEK, blocks FGF18 induction of MyoD (Figure 1C). Similarly grafting a U0126 bead at HH stage 21 and looking for MyoD expression after 24 hr reduces the endogenous expression of MyoD compared with the untreated contralateral limb (Figure 1D, E).
In limb buds at HH stage 19 FGF18 does not induce MyoD (Figure 1F). However co-grafting of beads soaked in FGF18 and the retinoic acid antagonist BMS493 can overcome this and ectopic MyoD is detected (Figure 1G)
To confirm that FGF18 is acting through MEK embryos were harvested 1 hr after bead graft and immunostained for phosphorylated ERK, the target of MEK. Figure 1H shows a brightfield image of an FGF18 bead 1 hr after grafting while Figure 1I shows that FGF18 induces rapid phosphorylation of ERK around the grafted bead. Figure 1J shows an overlay of these images. Control beads soaked in 0.1% BSA do not induce ERK phosphorylation (Figure 1K, L, M)
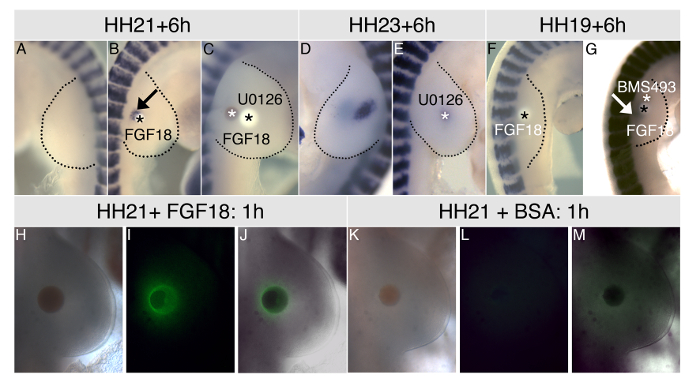
Figure 1. MyoD expression in limbs is regulated by FGF18 and retinoic acid through ERK phosphorylation. Unmanipulated control limbs at HH stage 21 do not express MyoD (A) but premature expression is detected 6 hr after grafting an FGF18 bead (B). Co-grafting FGF18 and U0126 beads blocks this induction (C). Endogenous MyoD expression is seen at HH stage 21 (D) but this is blocked by grafting beads soaked in U0126 (E). In earlier limb buds premature MyoD expression is not induced by FGF18 (F) but is induced by co-grafting beads soaked in FGF18 and the retinoic acid antagonist BMS493 (G). FGF18 beads grafted into HH21 limbs induce ERK phosphorylation after 1 hr: brightfield (H), fluorescent (I) and merged (J) images of an HH21 limb immunostained for anti-phosphoERK. Control beads soaked in BSA do not induce ERK phosphorylation: (K), fluorescent (L) and merged (M) images of an HH21 limb immunostained for anti-phosphoERK. This figure has been modified from Mok et al, 201416. Black asterisks: heparin beads; white asterisks: AG 1-X2 beads; arrows show ectopic MyoD expression in limb buds. Please click here to view a larger version of this figure.
Discussion
The use of bead grafts applied directly to developing tissues in ovo is a powerful tool to dissect the role of growth factor signaling during development giving unparalleled control over the developmental stage at which they are applied and the duration of exposure.
The choice of bead for each type of molecule is important. Small hydrophobic molecules, such as the inhibitors described here and retinoic acid, usually bind well to derivatized AG 1-X2 beads although it is necessary to test the effectiveness of each inhibitor on these beads empirically. Similarly, for growth factors it may be necessary to test different types of bead. Some beads are transparent and so can be hard to visualize during the manipulations but a short treatment with phenol red followed by a rinse in PBS will label them for long enough to perform the graft.
When preparing beads, particularly those which have been soaked with inhibitors, it is important to protect them from light as far as possible. During their initial treatment and during manipulation they should be covered and only removed when beads are being transferred. It is best to use freshly thawed aliquots as some of these reagents are unstable and can give misleading negative results if not stored properly. It is also important not to leave them soaking in phenol red for too long before grafting as this can reduce the effectiveness of the bead.
The concentrations used for these bead grafts, of both growth factors and drugs, are often very high; FGF18 beads are soaked in a concentration of 0.5 mg/ml and beads for small molecule inhibitors are often soaked at a concentration of 100 µM. Although this is much higher than the concentrations typically used to treat cells in solution this is necessary as the amounts of growth factors and drugs absorbed by beads and then released are hard to measure directly but presumably generate lower levels than this in vivo. As a result a range of concentrations must be tested for each molecule used to determine an effective dose.
This technique is also applicable to a wide range of other tissues, such as somites24, during development and can be used on embryos in ovo or in cultures, such as EC culture25. It is also possible to grow cultured cells which secrete specific growth factors as pellets which can then be grafted into developing embryos in the same way26.
The combination of accessibility, ease of manipulation and low cost makes chicken embryos an excellent model of development. As transgenic technologies are increasingly applied to avian species and genetically modified lines of both chickens7 and quails27 become available the combination of classical embryo grafting techniques with these modified birds is already providing novel insights into development8,28.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was partly funded by a University of Nottingham Early Career award to DS. RM is funded by the Higher Committee for Education Development of Iraq.
Materials
| Heparin acrylic beads | Sigma | H5263 | The acrylic-heparin beads used have been discontinued. However a replacement product is available, Heparin agarose beads, cat no H6508. These are transparent so harder to work with but can be stained with phenol red in the same way as AG 1-X2 beads, |
| AG 1-X2 beads | Bio-Rad | 140-1231 | |
| Affi Gel blue beads | Bio-Rad | 153-7301; 153-7302 | These beads have been used with a range of growth factors including Shh and FGFs and can be used to replace heparin beads |
| FGF18 | Peprotech | 100-28 | Resuspend in PBS with 0/1% BSA, prepare single use aliquots of 0.5-1ul and store at -80°C. Batches and suppliers can vary so different concentrations should be tested to determine an effective dose. |
| U0126 | Cell Signalling | 9903 | Make to 20mM stock in DMSO and store in single use aliquots at -80°C. Protect from light. |
| BMS-493 | Tocris Biosciences | 3509 | Resuspend in DMSO and store in single use aliquots at -80°C. Protect from light. |
| Black Indian ink | Windsor and Newton | 5012572003384 (30ml) | While alternatives to this product are available care should be taken as some inks are toxic to embryos |
| Tungsten wire, 0.1mm dia. 99.95% | Alfa Aesar | 10404 | |
| Penicillin / streptomycin | Sigma | P0781 | Dilute 100X in PBS/ink and PBS/FCS |
References
- Davey, M. G., Tickle, C. The chicken as a model for embryonic development. Cytogenetic Genome Research. 117, 231-239 (2007).
- Le Douarin, N. The Nogent Institute–50 years of embryology. International Journal of Developmental Biology. 49, 85-103 (2005).
- Le Douarin, N. M. The avian embryo as a model to study the development of the neural crest: a long and still ongoing story. Mechanisms of Development. 121, 1089-1102 (2004).
- Bell, E., Brickell, P. Replication-competent retroviral vectors for expressing genes in avian cells in vitro and in vivo. Molecular Biotechnology. 7, 289-298 (1997).
- Abu-Elmagd, M., et al. Wnt/Lef1 signaling acts via Pitx2 to regulate somite myogenesis. 발생학. 337, 211-219 (2010).
- Munsterberg, A. E., Lassar, A. B. Combinatorial signals from the neural tube, floor plate and notochord induce myogenic bHLH gene expression in the somite. Development. 121, 651-660 (1995).
- McGrew, M. J., et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Reports. 5, 728-733 (2004).
- Valasek, P., et al. Cellular and molecular investigations into the development of the pectoral girdle. 발생학. 357, 108-116 (2011).
- Tickle, C. The contribution of chicken embryology to the understanding of vertebrate limb development. Mechanisms of Development. 121, 1019-1029 (2004).
- Riddle, R. D., Johnson, R. L., Laufer, E., Tabin, C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 75, 1401-1416 (1993).
- Tickle, C., Alberts, B., Wolpert, L., Lee, J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 296, 564-566 (1982).
- Rosello-Diez, A., Torres, M. Regulative patterning in limb bud transplants is induced by distalizing activity of apical ectodermal ridge signals on host limb cells. Developmental Dynamics. 240, 1203-1211 (2011).
- Anakwe, K., et al. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development. 130, 3503-3514 (2003).
- Amthor, H., Christ, B., Weil, M., Patel, K. The importance of timing differentiation during limb muscle development. Current Biology. 8, 642-652 (1998).
- Brand-Saberi, B., Muller, T. S., Wilting, J., Christ, B., Birchmeier, C. Scatter factor/hepatocyte growth factor (SF/HGF) induces emigration of myogenic cells at interlimb level in vivo. 발생학. 179, 303-308 (1996).
- Mok, G. F., Cardenas, R., Anderton, H., Campbell, K. H. S., Sweetman, D. Interactions between FGF18 and retinoic acid regulate differentiation of chick embryo limb myoblasts. 발생학. 396, 214-223 (2014).
- Abou-Elhamd, A., Cooper, O., Münsterberg, A. Klhl31 is associated with skeletal myogenesis and its expression is regulated by myogenic signals and Myf-5. Mechanisms of Development. , 126-852 (2009).
- Schmidt, M., Tanaka, M., Munsterberg, A. Expression of (beta)-catenin in the developing chick myotome is regulated by myogenic signals. Development. 127, 4105-4113 (2000).
- Stavridis, M. P., Lunn, J. S., Collins, B. J., Storey, K. G. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 134, 2889-2894 (2007).
- Martinez-Morales, P. L., et al. FGF and retinoic acid activity gradients control the timing of neural crest cell emigration in the trunk. Journal of Cell Biology. 194, 489-503 (2011).
- Olivera-Martinez, I., Storey, K. G. Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development. 134, 2125-2135 (2007).
- Logan, M., Simon, H. G., Tabin, C. Differential regulation of T-box and homeobox transcription factors suggests roles in controlling chick limb-type identity. Development. 125, 2825-2835 (1998).
- Borycki, A. G., Mendham, L., Emerson, C. P. Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development. 125, 777-790 (1998).
- Sweetman, D., et al. FGF-4 signaling is involved in mir-206 expression in developing somites of chicken embryos. Developmental Dynamics. 235, 2185-2191 (2006).
- Chapman, S. C., Collignon, J., Schoenwolf, G. C., Lumsden, A. Improved method for chick whole-embryo culture using a filter paper carrier. Developmental Dynamics. 220, 284-289 (2001).
- Sweetman, D., Wagstaff, L., Cooper, O., Weijer, C., Münsterberg, A. The migration of paraxial and lateral plate mesoderm cells emerging from the late primitive streak is controlled by different Wnt signals. BMC Developmental Biology. 8, 63 (2008).
- Scott, B. B., Lois, C. Generation of tissue-specific transgenic birds with lentiviral vectors. Proceedings of the National Academy of Sciences of the U.S.A. 102, 16443-16447 (2005).
- Seidl, A. H., et al. Transgenic quail as a model for research in the avian nervous system: a comparative study of the auditory brainstem. Journal of Comparative Neurology. 521, 5-23 (2013).

