In ovo Expression of MicroRNA in Ventral Chick Midbrain
Summary
Ectopic expression is one technique to elucidate the microRNAs role in brain development. However, targeting specific areas using in ovo electroporation is challenging. Here, we show an efficient way to selectively electroporate ventral and dorsal midbrain regions.
Abstract
Non-coding RNAs are additional players in regulating gene expression. Targeted in ovo electroporation of specific areas provides a unique tool for spatial and temporal control of ectopic microRNA expression. However, ventral brain structures like ventral midbrain are rather difficult to reach for any manipulations. Here, we demonstrate an efficient way to electroporate miRNA into ventral midbrain using thin platinum electrodes. This method offers a reliable way to transfect specific areas of the midbrain and a useful tool for in vivo studies.
Introduction
The recognition of small non-coding RNAs as additional players for gene expression launched a new complexity to genomic programming/gene regulation. Different species of non-coding RNAs have functional importance in neural cells, including small non-coding RNAs1-4. MicroRNAs (miR or miRNA) for example show distinct and changing expression profiles in developing brains5. Targeted in ovo electroporation of chick embryos provides a unique opportunity for temporal and spatial control of gene expression and silencing during development.
This video demonstrates the different steps of performing ectopic expression of miRs in specific areas of the chick midbrain using in ovo electroporation6-10. To ensure a long lasting effect of these small non-coding RNAs in cells, the DNA sequence of miRs were cloned into mono- or bi-cistronic vectors. For in ovo electroporation, miR containing vector is injected into the midbrain neural tube by exposing the embryo after making a small window in the egg shell. To transfect specific areas of the midbrain small plus (anode) and minus (cathode) platinum electrodes are placed at specific positions. For ventral midbrain transfection, the anode is placed underneath the left ventral midbrain and the cathode above the right half of the midbrain before applying a current. The opening in the eggshell is closed with tape and embryos are incubated for as long as required for any analysis. This method was originally described by Muramatsu et al.6 and improved by Momose et al.8 for specific area transfection.
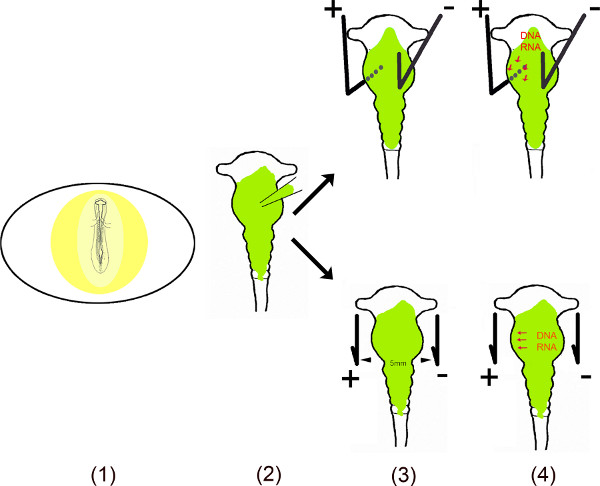
Schematic Overview.
- The embryo in the egg is exposed by cutting a small window into the eggshell.
- The dissolved vector(s) is injected into the midbrain using a micro capillary.
- Two electrodes – placed parallel or under and above the embryo – generate a pulsed electric field.
- The electric field temporally creates pores in the cell membrane, which facilitate entry into the cell by the negatively charged DNA (or RNA) attracted to the anode11,12.
Protocol
Representative Results
Discussion
This video demonstrates an effective method to transfect plasmid into the neuroepithelial cells of specific areas of the chick midbrain. Rectangular electric pulses of low voltage can introduce DNA into cells of the chick neural tube in ovo6,16. However, the accuracy of DNA targeting is often hindered by the wide electric field, which rises through the relatively large electrodes (Φ = 0.5 mm). We tried to tackle that problem by using electrodes smaller in diameter following the guidelines of Momos…
Disclosures
The authors have nothing to disclose.
Acknowledgements
We acknowledge K. Mikic, who contributed to the initial phase of this movie and M. Nicolescu for the miR picture. C. Huber was supported by a fellowship of the IZKF of the Universtitätsklinikum Tübingen, A. Alwin Prem Anand by the Fortüne programme of the Universtitätsklinikum Tübingen.
Materials
| Name | Company | Model | |
| Borosillicate glass capillaries | Hartenstein | Model: 0.9 mm | |
| Microcapillary puller | WPI, Berlin | Model: Pul1-E | |
| Electroporator | Intracel | Model: TSSIC | |
| Stereomicroscope – fluorescence | LEICA | Model: MZFLIII | |
| Stereomicroscope | Zeiss | Model: Stemi | |
| Camera and software | Zeiss | Model: Axiocam MRc/ Axiovision Re. 4.8 |
References
- Kutter, C., Svoboda, P. miRNA, siRNA, piRNA: Knowns of the unknown. RNA Biol. 5, 181-188 (2008).
- Szell, M., Bata-Csorgo, Z., Kemeny, L. The enigmatic world of mRNA-like ncRNAs: their role in human evolution and in human diseases. Semin. Cancer Biol. 18, 141-148 (2008).
- Li, X., Jin, P. Roles of small regulatory RNAs in determining neuronal identity. Nature reviews. Neuroscience. 11, 329-338 (2010).
- Riedmann, L. T., Schwentner, R. miRNA, siRNA, piRNA and argonautes: news in small matters. RNA Biol. 7, 133-139 (2010).
- Coolen, M., Bally-Cuif, L. MicroRNAs in brain development and physiology. Curr. Opin. Neurobiol. 19, 461-470 (2009).
- Muramatsu, T., Mizutani, Y., Ohmori, Y., Okumura, J. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochem. Biophys. Res. Commun. 230, 376-380 (1997).
- Itasaki, N., Bel-Vialar, S., Krumlauf, R. Shocking’ developments in chick embryology: electroporation and in ovo gene expression. Nat. Cell Biol. 1, 203-207 (1999).
- Momose, T., et al. Efficient targeting of gene expression in chick embryos by microelectroporation. Dev. Growth Differ. 41, 335-344 (1999).
- Nakamura, H., Watanabe, Y. Misexpression of genes in brain vesicles by in ovo electroporation. Dev. Growth Differ. 42, 199-201 (2000).
- Voiculescu, O., Papanayotou, C., Stern, C. D. Spatially and temporally controlled electroporation of early chick embryos. Nat. Protoc. 3, 419-426 (2008).
- Neumann, E., Schaefer-Ridder, M., Wang, Y., Gene Hofschneider, P. H. transfer into mouse lyoma cells by electroporation in high electric fields. The EMBO journal. 1, 841-845 (1982).
- Potter, H., Weir, L., Leder, P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proceedings of the National Academy of Sciences of the United States of America. 81, 7161-7165 (1984).
- Krull, C. E. A primer on using in ovo electroporation to analyze gene function. Dev. Dyn. 229, 433-439 (2004).
- Canto-Soler, M. V., Adler, R. Optic cup and lens development requires Pax6 expression in the early optic vesicle during a narrow time window. Developmental biology. , 294-2119 (2006).
- Hamburger, V., Hamilton, H. L. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 195, 231-272 (1992).
- Muramatsu, T., Nakamura, A., Park, H. M. In vivo electroporation: a powerful and convenient means of nonviral gene transfer to tissues of living animals (Review). Int J Mol Med. 1, 55-62 (1998).
- Agoston, Z., Li, N., Haslinger, A., Wizenmann, A., Schulte, D. Genetic and physical interaction of Meis2, Pax3 and Pax7 during dorsal midbrain development. BMC Dev Biol. 12, 10 (2012).
- De Pietri Tonelli, D., et al. Single-cell detection of microRNAs in developing vertebrate embryos after acute administration of a dual-fluorescence reporter/sensor plasmid. Biotechniques. 41, 727-732 (2006).

