多峰成像和光谱学纤维束显微内窥镜纲要非侵入性,<em>在体内</em>组织分析
Summary
The assembly and use of a multimodal microendoscope is described which can co-register superficial tissue image data with tissue physiological parameters including hemoglobin concentration, melanin concentration, and oxygen saturation. This technique can be useful for evaluating tissue structure and perfusion, and can be optimized for individual needs of the investigator.
Abstract
最近纤维束显微内窥镜技术使用两种成像技术或分光技术的组合在体内组织的非侵入性的分析。结合成像和光谱学技术成一个单一的光学探针可提供组织健康的更完整的分析。在这篇文章中,两种不同的方式组合,高分辨率荧光显微内窥镜成像和漫反射光谱,成一个单一的光学探头。高分辨率荧光显微内窥镜成像是用于可视化根尖组织微结构的技术,虽然大多是定性的技术,已经证明肿瘤和非肿瘤组织之间的有效实时分化。漫反射光谱是一种技术,它可以提取组织的生理参数,包括当地的血红蛋白浓度,黑色素浓度和血氧饱和度。本文介绍的规格řequired构建光纤探头,如何建立仪器仪表,然后演示了在体内对人体皮肤的技术。这项工作表明该组织的微架构,特别是心尖皮肤角质,可以和其相关的生理参数共同注册。这里介绍的仪器和纤维束探针可以被优化为在各种器官系统的使用任一手持或内窥镜兼容设备。另外的临床研究来测试此技术对于不同的上皮疾病状态的可行性。
Introduction
纤维束显微内窥镜技术通常使用两种成像技术或分光技术的组合分析体内组织中 1-3一种这样的成像技术,高分辨率荧光显微内窥镜,可以形象根尖组织微架构具有亚细胞分辨率的小,微尺度场的图,利用局部造影剂如原黄素,荧光素,或羟基芘磺酸油墨。1,3-11该成像模态已示于定性实时低区分患病的和健康上皮组织有前途的临床表现间观察员变异8偶尔,调查人员将使用高分辨率荧光显微镜的数据中提取定量的功能,如细胞与核的大小或腺区,但这仍然瞄准往可视化的组织形态的主要定性技术。1,3,8- 10在另一方面,光谱技术等作为漫反射光谱,正朝着提供功能组织信息并显示在多个器官定量识别病变癌变看好的临床表现有针对性的。2,12-15
因此,有必要对掺入两种类型的模式,从而可能进一步降低观察者间变异性,维持组织微架构的实时可视化,并提供组织健康的一个更完整的分析的装置。为了实现这个目标,在基于探针的多峰仪器构建结合在一个单一的光纤探针两种模式:高分辨率荧光显微内窥镜和分漫反射光谱11此方法共寄存器定性心尖的高清晰度图像组织形态(结构特性)与来自两个不同的组织深度定量光谱信息(功能性),包括局部血红蛋白浓度([血红蛋白]),黑色素浓度([梅尔])和氧饱和度(SAO 2)。11,12,16这个特定副漫反射光谱形态使用两个源-检测器分离(SDSS)采样两个独特的组织深度,以提供通过采样到地下室膜和皮下组织间质组织健康更全面的了解。11
纤维探头由具有大约50,000 4.5微米直径的纤维元件,1.1mm的包层直径和1.2mm的整体涂层直径的中央毫米直径的1个图像的纤维。图像纤维是通过用220微米的包层直径5 200微米直径的纤维包围。每200μm多模光纤位于中心到中心的距离864微米从图像光纤的中心了。每200微米的多模光纤的相隔25°。使用最左边的200μm多模光纤为“源”的纤维,以及附加次稀土元素200μm多模光纤为“收藏”的纤维,这种几何必然创建三个中心到中心的科学数据集的374微米,730微米,1051微米,1323微米。纤维尖端封闭在保持纤维常数之间的距离的筒状金属外壳。圆筒形金属外壳的直径为3毫米。前端(朝向光纤探针尖端)的光纤探针的为2英尺长。探针然后分离成在近端(朝向仪表)的六个相应的各个纤维是额外的2英尺长,对于4英尺总长度。 图1示出了光纤探针的表示。
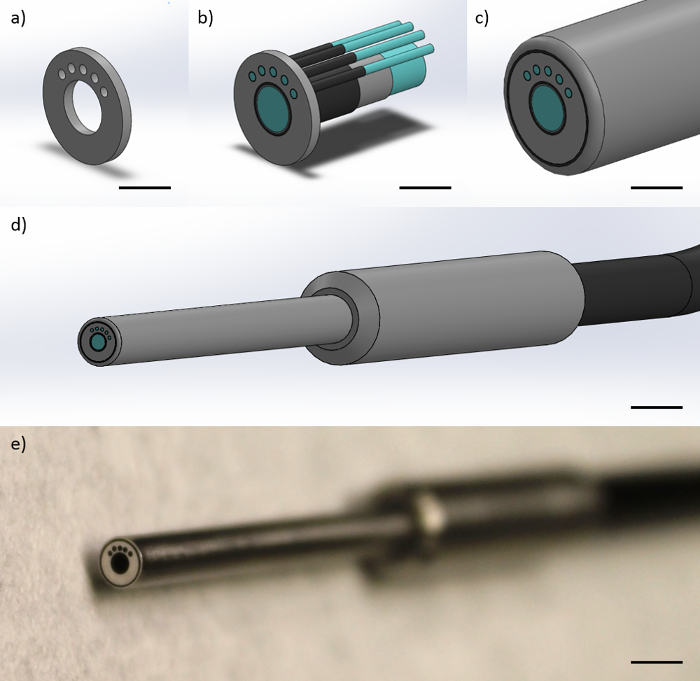
图1:光纤探头设计的光纤探针包括一个毫米直径的1图像纤维和四个200微米的多模光纤。这个图中显示的表示(a)该金属端盖这限制了纤维的几何形状在探头尖端,得到的安全数据表374,730,和1 051微米相对于最左边的200微米的多模光纤(比例尺≈1毫米), (b)该纤维被约束在金属盖内,示出了纤维芯,光纤包层,和纤维涂层(比例尺≈1毫米),(C)周围的纤维保护聚酰胺护套(比例尺≈1毫米),( 四 )成品远侧尖端的探头,与金属手指抓握和含有所有纤维单黑线(比例尺≈4毫米),和(e)的探针(比例尺≈为4mm远侧尖端的一个图片)。 请点击此处查看该图的放大版本。
这种多模式的仪器和相关TECHNI阙是一个探头内的这些方式的第一组合,但也存在其他组合结构/功能技术,结合不同的方式。例如,高光谱成像结合定量血红蛋白和黑色素特性,17,18和其它技术已经开发了与组织蛋白表达的分析,19结合光学相干断层扫描(OCT),仅举几宽视场成像。对使用的一般的光纤探针可用于各种目的,包括在口腔中的下胃肠道和食道或作为手持式探头内窥镜用途的使用进行优化的紧凑和易于实施的仪器设置本文报告和外部皮肤位置。11,20
该仪器的硬件既需要自定义的数据采集和处理后的代码来获得漫反射光谱,然后提取所产生的volumE-平均组织的生理参数,包括[血红蛋白],[梅尔]和血氧饱和。定制数据获取代码的建立是为了允许来自摄像头的同时采集(高分辨率荧光显微镜)和一个分光计(对于漫反射光谱)。驱动程序通常可从制造商的网站以允许与各种编程语言的融合。定制后处理代码出口先验吸收值的体内 [血红蛋白]和[梅尔] 21,然后利用创建该光谱的拟合曲线一个先前开发的非线性优化拟合过程22的拟合曲线是通过最小化建本身和原光谱和之间χ2值从拟合曲线并具有最低χ2值22的代码确定该组织的生理参数([血红蛋白],[梅尔]和血氧饱和2)可修改成包括吸收来自其他发色为好,如这里使用的外源性羟基芘磺酸墨水,使目标生理参数均不受影响。
组织健康的生理指标,如[血红蛋白],[梅尔]和血氧饱和,可作为肿瘤对治疗的反应的报告或本地血管和血管生成的指标。14,23包括一个高分辨率荧光显微内窥镜形态有助于引导探头的位置,并提供与调查上皮组织结构和功能之间的关系更完整的画面。在这篇文章中,建设和多式联运显微内窥镜的应用说明。11
Protocol
Representative Results
Discussion
多峰高分辨率成像和分漫反射光谱纤维束显微内窥镜此处报告可以被优化并用于各种应用,包括内窥镜或手持使用用于人类或动物研究中使用由调查员。因此,它提供了用于在体内根尖组织微结构可视化与来自两个不同的组织深度的血红蛋白浓度,黑色素浓度和组织氧饱和度的测量值的灵活的方法。本文介绍了用于光纤探针的说明书,所述的协议用于组装高分辨率成像系统和分漫反射?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
This material is based on work supported by the National Institutes of Health (1R03-CA182052, 1R15-CA202662), the National Science Foundation Graduate Research Fellowship Program (G.G., DGE-1450079), the Arkansas Biosciences Institute, and the University of Arkansas Doctoral Academy Fellowship. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the acknowledged funding agencies.
Materials
| 30 mm Cage Cube with Dichroic Filter Mount | Thorlabs, Inc. | CM1-DCH | |
| 470 nm Dichroic Mirror (Beam Splitter) | Chroma Corporation | T470lpxr | |
| Cage Assembly Rod, 1.5", 4-Pack | Thorlabs, Inc. | ER1.5-P4 | |
| Cage Assembly Rod, 3.0", 4-Pack | Thorlabs, Inc. | ER3-P4 | |
| Cage Assembly Rod, 2.0", 4-Pack | Thorlabs, Inc. | ER2-P4 | |
| SM1-Threaded 30 mm Cage Plate | Thorlabs, Inc. | CP02 | |
| SM1 Series Stress-Free Retaining Ring | Thorlabs, Inc. | SM1PRR | |
| SM1 Lens Tube, 1.00" Thread Depth | Thorlabs, Inc. | SM1L10 | |
| Right-Angle Kinematic Mirror Mount | Thorlabs, Inc. | KCB1 | |
| 1" UV Enhanced Aluminum Mirror | Thorlabs, Inc. | PF10-03-F01 | |
| Z-Axis Translation Mount | Thorlabs, Inc. | SM1Z | |
| 10X Olympus Plan Achromatic Objective | Thorlabs, Inc. | RMS10X | |
| XY Translating Lens Mount | Thorlabs, Inc. | CXY1 | |
| SMA Fiber Adapter Plate with SM1 Thread | Thorlabs, Inc. | SM1SMA | |
| SM1 Lens Tube, 0.50" Thread Depth | Thorlabs, Inc. | SM1L05 | |
| 440/40 Bandpass Filter (Excitation) | Chroma Corporation | ET440/40x | |
| 525/36 Bandpass Filter (Emission) | Chroma Corporation | ET525/36m | |
| Quick Set Epoxy | Loctite | 1395391 | |
| 455 nm LED Light Housing Kit – 3-Watt | LED Supply | ALK-LH-3W-KIT | |
| 1" Achromatic Doublet, f=50mm | Thorlabs, Inc. | AC254-050-A | |
| Flea 3 USB Monochrome Camera | Point Grey, Inc. | FL3-U3-32S2M-CS | |
| 0.5" Post Holder, L = 1.5" | Thorlabs, Inc. | PH1.5 | |
| 0.5" Optical Post, L = 4.0" | Thorlabs, Inc. | TR4 | |
| Mounting Base, 1" x 2.3" x 3/8" | Thorlabs, Inc. | BA1S | |
| Long Lifetime Tungsten-Halogen Light Source (Vis-NIR) | Ocean Optics | HL-2000-LL | |
| 20X Olympus Plan Objective | Edmund Optics, Inc. | PLN20X | |
| Custom-Built Aluminum Motor Arm | N/A | N/A | Custom designed and built |
| Custom-Built Aluminum Motor Arm Adaptor | N/A | N/A | Custom designed and built |
| Custom-Built Aluminum Motor Housing | N/A | N/A | Custom designed and built |
| Stepper Motor – 400 steps/revolution | SparkFun Electronics | ROB-10846 | Multiple suppliers |
| Custom-Built Aluminum Optical Fiber Switch | N/A | N/A | Custom designed and built |
| Custom-Built Aluminum Optical Fiber Switch Face-Plate | N/A | N/A | Custom designed and built |
| Arduino Uno – R3 | SparkFun Electronics | DEV-11021 | Multiple suppliers |
| Electronic Breadboard – Self-Adhesive | SparkFun Electronics | PRT-12002 | Multiple suppliers |
| EasyDriver – Stepper Motor Driver | Sparkfun Electronics | ROB-12779 | |
| 12V, 229 mA Power Supply | Phihong | PSM03A | Multiple suppliers |
| Enhanced Sensitivity USB Spectrometer (Vis-NIR) | Ocean Optics | USB2000+VIS-NIR-ES | |
| 550 µm, 0.22 NA, SMA-SMA Fiber Patch Cable | Thorlabs, Inc. | M37L01 | |
| Custom-Built Fiber-Optic Probe | Myriad Fiber Imaging | N/A | |
| 20% Spectralon Diffuse Reflectance Standard | Labsphere, Inc. | SRS-20-010 | |
| Standard Yellow Highlighter | Sharpie | 25005 | Multiple suppliers, proflavine or fluorescein can be substituted |
References
- Muldoon, T. J., et al. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 15, 16413-16423 (2007).
- Rajaram, N., Reichenberg, J. S., Migden, M. R., Nguyen, T. H., Tunnell, J. W. Pilot clinical study for quantitative spectral diagnosis of non-melanoma skin cancer. Lasers Surg Med. 42, 716-727 (2010).
- Louie, J. S., Richards-Kortum, R., Anandasabapathy, S. Applications and advancements in the use of high-resolution microendoscopy for detection of gastrointestinal neoplasia. Clin Gastroenterol Hepatol. 12, 1789-1792 (2014).
- Chang, S. S., et al. High resolution microendoscopy for classification of colorectal polyps. Endoscopy. 45, 553-559 (2013).
- Muldoon, T. J., et al. Noninvasive imaging of oral neoplasia with a high-resolution fiber-optic microendoscope. Head Neck. 34, 305-312 (2011).
- Muldoon, T. J., et al. Evaluation of quantitative image analysis criteria for the high-resolution microendoscopic detection of neoplasia in Barrett’s esophagus. J Biomed Opt. 15, 026027 (2010).
- Prieto, S. P., Powless, A. J., Boice, J. W., Sharma, S. G., Muldoon, T. J. Proflavine Hemisulfate as a Fluorescent Contrast Agent for Point-of-Care Cytology. PLoS One. 10, e0125598 (2015).
- Parikh, N., et al. In vivo diagnostic accuracy of high resolution microendoscopy in differentiating neoplastic from non-neoplastic colorectal polyps: a prospective study. Am J Gastroenterol. 109, 68-75 (2014).
- Shin, D., et al. Quantitative analysis of high-resolution microendoscopic images for diagnosis of esophageal squamous cell carcinoma. Clin Gastroenterol Hepatol. 13, 272-279 (2015).
- Prieto, S. P., et al. Qualitative and quantitative comparison of colonic microendoscopy image features to histopathology. Proc SPIE Int Soc Opt Eng. 9328, (2015).
- Greening, G. J., et al. Fiber-bundle microendoscopy with sub-diffuse reflectance spectroscopy and intensity mapping for multimodal optical biopsy of stratified epithelium. Biomed Opt Express. 6, 4934-4950 (2015).
- Rajaram, N., Gopal, A., Zhang, X., Tunnell, J. W. Experimental validation of the effects of microvasculature pigment packaging on in vivo diffuse reflectance spectroscopy. Lasers Surg Med. 42, 680-688 (2010).
- Spliethoff, J. W., et al. Monitoring of tumor response to cisplatin using optical spectroscopy. Transl Oncol. 7, 230-239 (2014).
- Chang, V. T., et al. Quantitative physiology of the precancerous cervix in vivo through optical spectroscopy. Neoplasia. 11, 325-332 (2009).
- Yu, B., Shah, A., Nagarajan, V. K., Ferris, D. G. Diffuse reflectance spectroscopy of epithelial tissue with a smart fiber-optic probe. Biomed Opt Express. 5, 675-689 (2014).
- Hennessy, R., Goth, W., Sharma, M., Markey, M. K., Tunnell, J. W. Effect of probe geometry and optical properties on the sampling depth for diffuse reflectance spectroscopy. J Biomedical Opt. 19, 107002 (2014).
- Ghassemi, P., Travis, T. E., Moffatt, L. T., Shupp, J. W., Ramella-Roman, J. C. A polarized multispectral imaging system for quantitative assessment of hypertrophic scars. Biomed Opt Express. 5, 3337-3354 (2014).
- Vasefi, F., et al. Polarization-sensitive hyperspectral imaging in vivo: a multimode dermoscope for skin analysis. Sci Rep. 4, (2014).
- Winkler, A. M., Rice, P. F. S., Drezek, R. A., Barton, J. K. Quantitative tool for rapid disease mapping using optical coherence tomography images of azoxymethane-treated mouse colon. J Biomedl Opt. 15, 041512 (2010).
- Bish, S. F., et al. Handheld Diffuse Reflectance Spectral Imaging (DRSi) for in-vivo characterization of skin. Biomed Opt Express. 5, 573-586 (2014).
- Prahl, S. A. . Optical Absorption of Hemoglobin. , (1999).
- Rajaram, N., et al. Design and validation of a clinical instrument for spectral diagnosis of cutaneous malignancy. Appl Opt. 49, 142-152 (2010).
- Hennessy, R., Markey, M. K., Tunnell, J. W. Impact of one-layer assumption on diffuse reflectance spectroscopy of skin. J Biomed Opt. 20, 27001 (2015).
- Rajaram, N., Nguyen, T. H., Tunnell, J. W. Lookup table-based inverse model for determining optical properties of turbid media. J Biomed Opt. 13, 050501 (2008).
- Nichols, B. S., Rajaram, N., Tunnell, J. W. Performance of a lookup table-based approach for measuring tissue optical properties with diffuse optical spectroscopy. J Biomed Opt. 17, 057001 (2012).
- Greening, G. J., James, H. M., Muldoon, T. J. . Optical Phantoms: Diffuse and Sub-diffuse Imaging and Spectroscopy Validation. , 1-37 (2015).
- Karsten, A. E., Smit, J. E. Modeling and verification of melanin concentration on human skin type. Photochem Photobiol. 88, 469-474 (2012).
- Glennie, D. L., Hayward, J. E., Farrell, T. J. Modeling changes in the hemoglobin concentration of skin with total diffuse reflectance spectroscopy. J Biomed Opt. 20, 035002 (2015).
- Lim, L., Nichols, B., Rajaram, N., Tunnell, J. W. Probe pressure effects on human skin diffuse reflectance and fluorescence spectroscopy measurements. J Biomed Opt. 16, 011012 (2011).

