In Vivo Wireless Optogenetic Control of Skilled Motor Behavior
Summary
The present protocol describes how to use wireless optogenetics combined with high-speed videography in a single pellet reach-to-grasp task to characterize the neural circuits involved in the performance of skilled motor behavior in freely moving mice.
Abstract
Fine motor skills are essential in everyday life and can be compromised in several nervous system disorders. The acquisition and performance of these tasks require sensory-motor integration and involve precise control of bilateral brain circuits. Implementing unimanual behavioral paradigms in animal models will improve the understanding of the contribution of brain structures, like the striatum, to complex motor behavior as it allows manipulation and recording of neural activity of specific nuclei in control conditions and disease during the performance of the task.
Since its creation, optogenetics has been a dominant tool for interrogating the brain by enabling selective and targeted activation or inhibition of neuronal populations. The combination of optogenetics with behavioral assays sheds light on the underlying mechanisms of specific brain functions. Wireless head-mounted systems with miniaturized light-emitting diodes (LEDs) allow remote optogenetic control in an entirely free-moving animal. This avoids the limitations of a wired system being less restrictive for animals’ behavior without compromising light emission efficiency. The current protocol combines a wireless optogenetics approach with high-speed videography in a unimanual dexterity task to dissect the contribution of specific neuronal populations to fine motor behavior.
Introduction
Motor skilled behavior is present during most movements performed by us, and it is known to be affected in several brain disorders1,2,3,4,5,6. Implementing tasks that allow studying the development, learning, and performance of skilled movements is crucial to understanding the motor function's neurobiological underpinnings, especially in models of brain injury, neurodegenerative and neurodevelopmental disorders2,7,8,9,10,11,12,13. Reaching for and retrieving objects is done routinely in everyday life actions, and it is one of the first motor skills acquired during early development and then refined through the years5,6. It comprises a complex behavior that requires sensory-motor processes such as the perception of the object's features, movement planning, action selection, movement execution, body coordination, and speed modulation7,14,15,16. Thus, unimanual high dexterity tasks require the participation of many brain structures of both hemispheres16,17,18,19,20,21,22. In mice, the single pellet reach-to-grasp task is characterized for several phases that can be controlled and analyzed separately7,13,23. This feature allows to study the contribution of specific neuronal subpopulations at different stages of acquisition and behavior performance and provides a platform for detailed studies of motor systems13,23,24. The movement occurs in a couple of seconds; thus, high-speed videography should be used for kinematic analysis in distinct stages of the skilled motor trajectory7,25. Several parameters can be extracted from the videos, including body posture, trajectory, velocity, and type of errors25. Kinematic analysis can be used to detect subtle changes during wireless optogenetic manipulation7,23.
Using miniaturized light-emitting diodes (LEDs) to deliver light via a wireless head-mounted system makes it possible to have remote optogenetic control while the animal performs the task. The wireless optogenetic controller accepts single-pulse or continuous trigger commands from a stimulator and sends infrared (IR) signals to a receiver connected to the miniaturized LED23,26. The current protocol combines this wireless optogenetics approach with high-speed videography of a dexterity task to dissect the role of specific neuronal populations during the performance of fine motor behavior23. Since it is a unimanual task, it allows for assessing the participation of structures in both hemispheres. Traditionally, the brain controls the body movement in a highly asymmetric manner; however, high dexterity tasks require careful coordination and control from many brain structures, including ipsilateral nuclei and differential contribution of neuronal subpopulations within nuclei10,20,21,22,23. This protocol shows that subcortical structures from both hemispheres control the trajectory of the forelimb23. This paradigm can be suitable to study other brain regions and models of brain disease.
Protocol
The procedures involving animal use were conducted following local and national guidelines and approved by the corresponding Institutional Animal Care and Use Committee (Institute of Cellular Physiology IACUC protocol VLH151-19). Drd1-Cre transgenic male mice27, 35-40 days postnatal with C57BL/6 background were used in the current protocol. Mice were kept under the following conditions: temperature 22±1 °C; humidity 55%; light schedule 12/12 h with lights off at 7 p.m. and were weaned at postnatal day 21. Weaned pups were housed in same-sex groups of 2-5. Animals were housed in static housing with micro-barrier tops. Bedding consisted of sterile aspen shavings. Rodent pellets and RO-purified water were provided ad libitum, except when noted.
1. Surgical procedures
- Prepare an LED cannula at the desired length according to the dorsoventral coordinates of the structure of interest (ideally 0.5 mm longer to account for the thickness of the skull, for the dorsolateral striatum 3.5 mm) (Figure 1).
- Cut the glass fiber to a length longer than the final desired size, grind the fiber tip to the target length with rough sandpaper, and finally, polish the fiber tip with fine sandpaper.
NOTE: LED cannula is a glass optical fiber of 250 µm diameter attached to an infrared receiver (see Table of Materials).
- Cut the glass fiber to a length longer than the final desired size, grind the fiber tip to the target length with rough sandpaper, and finally, polish the fiber tip with fine sandpaper.
- Pull glass pipettes (1.14 mm outer diameter, 0.53 mm inner diameter, and 3.5 in length) for the nano-injector with a horizontal puller (see Table of Materials) and store them for later. Program the puller in one loop to get a 15-20 µm tip diameter with a long gradual slope taper (4-5 mm).
- Prepare the surgery area by thoroughly disinfecting the stereotaxic apparatus, hood, micro-injector (see Table of Materials), and surrounding surfaces with 70% ethanol.
NOTE: A mouse stereotaxic apparatus is essential to inject Adeno Associated Virus (AAVs) precisely and place the LED cannula in the region of interest. - Wear the appropriate personal protective equipment for the procedure, including a clean lab coat or disposable surgical gown, sterile gloves, face mask, and disposable head cap.
- Place the necessary equipment close to the surgery area, such as sterile surgical tools, cotton tips, solutions, micropipette, pipette tips, capillaries, micro-fill with mineral oil, and marker.
- Fill a pipette for microinjections with mineral oil and place it in the micro-injector. Make sure that the micro-injector is working correctly by ejecting some mineral oil.
NOTE: All instruments used during the surgery should be autoclaved and sterile. Aseptic technique should be used. - Anesthetize animals with gaseous isoflurane 4-5% to induce anesthesia and 1.2% throughout the surgery with 0.5-1 L/min pure oxygen. The surgery begins only after the animal has reached a point of deep anesthesia, assessed by the absence of paw withdrawal after a slight pinch.
- Continuously monitor the breathing rate and temperature of the animal. Maintain the body temperature by a heating pad set at 34 °C.
- Apply an ophthalmic ointment. Remove hair from the scalp with a trimer and hair removal cream. Wipe the scalp with cotton swabs having 8% povidone-iodine (see Table of Materials) and 70% ethanol alternated three times each.
- Place the mouse in the stereotaxic apparatus and secure the head, ensuring that the skull is leveled in the mediolateral and anterior-posterior axes.
- Make a 1 cm incision with a scalpel through the scalp at the level of the eyes along the sagittal axis. Retract the skin to expose the skull and clean the periosteum with cotton swabs.
- Clean the cranium surface with saline solution and sterile cotton swabs. Resolve any bleeding at the surface using sterile absorbent eye spears (see Table of Materials) or similar sterile absorbent material.
- Apply a drop of 2.5% hydrogen peroxide with a cotton swab and let it act for a few seconds to make the skull sutures visible and have a better reference. After a few seconds, clean thoroughly with a clean cotton swab.
- With the glass pipette (15 µm final tip diameter), locate bregma and lambda to check that the skull is leveled in the anterior-posterior axis.
NOTE: It is recommended to have a stereoscopic microscope or USB microscope to see the tip of the glass pipette. In case it is needed, adjust the height of the mouth holder to level the skull. - Move the capillary toward the selected anterior-posterior (AP) and medial-lateral (ML) coordinates (dorsolateral striatum AP 1.2 mm, ML 2.28 mm). Paint a reference point in the scalp above the selected coordinates with a sterile marker.
- In the reference point, perform an ~1 mm diameter craniotomy applying gentle pressure to the skull with a sterile rotary tool or dental drill at a low to medium speed with a small round dental drill bit (see Table of Materials).
- Load the capillary with 300-400 nL of Cre-dependent adeno-associated virus (AAV) such as AAV1-dflox-hChR-2-mCherry to express Channelrhodopsin or an AAV to express only the reporter protein (e.g., mCherry) as a control in the region of interest (see Table of Materials). Check that the tip is not clogged, then introduce the glass pipette in the brain at the desired dorso-ventral (DV) coordinates (dorsolateral striatum DV -3.35 mm).
- Inject 200 nL using an automatic injector at a rate of 23 nL/s. Wait for 10 min after finishing the injection, withdraw the glass pipette slowly to avoid spillage.
NOTE: It is possible to use a 30 G needle to inject with the appropriate micro-injector.
- Inject 200 nL using an automatic injector at a rate of 23 nL/s. Wait for 10 min after finishing the injection, withdraw the glass pipette slowly to avoid spillage.
- Clean and dry any residues with cotton swabs.
- Attach the sterile glass LED cannula to the stereotaxic arm and calibrate the coordinates using bregma as a reference. Insert the cannula very slowly (300 µm/min) to avoid tissue damage and place it 100 µm above the injection site.
- Once the LED cannula is in place, add a drop (100 µL) of tissue adhesive at the edge of the craniotomy.
- Prepare dental cement mixture (see Table of Materials) following the manufacturer's instructions to fix the fiber to the skull.
NOTE: Briefly, use a chilled porcelain dish to have more working time before cement sets. Add 2 scoops of resin clear powder to the porcelain dish, add 4 drops of quick base and 1 drop of catalyst, then mix well. The powder/liquid ratio can be adjusted if a thinner or thicker viscosity is needed. - Using a sterile brush, apply the dental cement mixture around the cannula connector little by little, building layers until the skull is covered and the connector is securely attached to the skull, leaving the pins completely free. Avoid getting dental cement on the skin of the mouse.
- Allow drying completely.
- Close the skin around the implant using tissue adhesive (see Table of Materials).
- Place the mouse in a recovery cage over a heating pad at 33° C. Monitor for the presence of one or more of the following signs of pain/discomfort: 1) Hunched up, lack or reduction of motor activity, 2) Failure to groom reflected in a unkept dirty coat, 3) Excessive licking or scratching, redness in the incision site, 4) aggressive behavior, 5) anorexia or dehydration, and 6) Lack of nest formation.
NOTE: Keep the mouse individually caged during all the procedures to avoid implant detaching. In case of detachment of the cannula, perform euthanasia by injecting 150 mg/kg of sodium pentobarbital followed by decapitation after deep anesthesia is reached. - Inject subcutaneously (SC) meloxicam 1 mg/kg once daily for three days post-surgery to provide analgesia.
- Wait at least 7 days for complete recovery and 14 days for opsin expression before further procedures.
NOTE: Perform a postoperative follow-up every 12 hours for three days, then check animals every day until the day of euthanasia at the end of the experiment.
2. Reach-to-grasp training
- On day 7 post-surgery, start the food deprivation protocol28. Weigh mice for three consecutive days to determine their average ad libitum body weight. Then, schedule food restrictions so that the animals receive enough nutrients to maintain approximately 90% and not less than 85% of body weight.
NOTE: This is achieved by providing 2.5-3 g of food daily. Monitor animals' weight daily and score for overall well-being observing animals' behavior and appearance, for example, coat and eyes appearance. Use the body condition scoring system from Reference29. - During the pre-training, training, and testing periods, provide each mouse with 20 pellets (20 mg of dustless chocolate-flavored pellets) daily (see Table of Materials) (eaten during the task or after) besides the standard food pellets.
- Three days before habituation, scatter 0.4 g/animal/day 20 mg of dustless chocolate-flavored pellets in their home cages, so mice get acquainted with the pellets that serve as a reward during the reach-to-grasp task.
- Habituate mice by placing them 10 min in the testing chamber one day before pre-training with pellets scattered on the chamber floor (Figure 1A).
- Allow food daily after training and testing. Keep a similar schedule every day.
- On the first day of pre-training, place the mice in the reach-to-grasp chamber and observe from the front. Place the pellets in front of the chamber close to the opening so that they start consuming the pellets. At this stage, mice are allowed to grab the pellets in any form.
- On day two of pre-training, place the pellets further and further from the opening until getting them to the indentation (1 cm from the opening) so mice can shape their reach-to-grasp movement (Figure 1C).
- Train mice to run to the rear of the cage and return to the cage opening to receive the next food pellet as a strategy to individualize trials.
NOTE: This can be achieved by waiting until the mouse is in the rear of the cage before placing a pellet in the indentation for each trial. - Place pellets to be grasped by either their right or left paw.
NOTE: Mice start using preferentially one paw to grasp, which will be used the following days of training and testing. - Train animals for 6 days in daily sessions lasting 20 trials or until a maximum of 10 min elapse. From day 2 of training, put the mock receiver (dimensions 12 x 18 x 7 mm, 1 g, see Table of Materials), so mice get habituated to the weight while performing the task (Figure 1B). Each day score the number of hit and missed trials.
- Record behavior with a regular camera and capture 30-60 frames/s from the front of the chamber. Additionally, one can place a mirror under the training chamber at a 45° angle to monitor the animals' posture (Figure 1D,E).
- For post-hoc kinematic analysis (Figure 2), mount a high-speed camera (see Table of Materials) at an angle of 45° to record from the side of the cage. If a 3D analysis is required, place a second high-speed camera to record at a 35° angle from the front of the chamber; both cameras should be placed in the right or left side of the cage depending on animals' sidedness and should capture at the same frame rate and be synchronized7 (Figure 3D,E).
- Set the high-speed cameras to 100 frames/s with a resolution of 376 x 252 pixels or more if possible. Place white Styrofoam walls behind the sides and back of the chamber to reduce background and increase contrast (Figure 1E).
- On test day, replace the mock unit with an infrared receiver for wireless optogenetic stimulation (Figure 1B,C).
- When mice start reaching, turn the LED cannula manually with the remote controller to have a continuous stimulation for the time the behavior is performed and for no longer than 2 s. Programming an automatic stimulation paradigm is preferable. The stimulation device triggers an LED of 470 nm (blue light) with intensity at the tip of 1.0 mW/mm2.
- Collect the videos for further examination, including scoring and kinematic analysis.
3. Post-hoc histological confirmation
- Upon completion of an experiment, confirm viral expression and LED cannula placement. Anesthetize the animal with a cocktail of ketamine 100 mg/kg and xylazine 10 mg/kg. Once the mouse presents signs of deep anesthesia (step 1.7), perfuse with ice-cold phosphate-buffered saline (PBS) followed by 4% PFA.
- Remove implanted cannula carefully by firmly grasping the connector with forceps and pulling up gently.
- Extract and post-fix the brain for 24 h in 4% PFA23.
- Perform 3-10 min washes with PBS.
- Cut the brain in 50 µm sections using a microtome (see Table of Materials).
- Mount the sections in slides with hard-set mounting media with DAPI to stain nuclei and cover slides.
- After drying, observe the sections under the confocal microscope and verify the implanted cannula location and expression of Ch2R fused with any fluorescent protein.
Representative Results
The reach-to-grasp task is a paradigm widely used to study shaping, learning, performance, and kinematics of fine skill movement under different experimental manipulations. Mice learn to execute the task in a couple of days and achieve more than 55% accuracy reaching a plateau after 5 days of training (Figure 2A,B). Similar to what has been previously reported, a percentage of animals do not perform the task appropriately (29.62%), and those should be excluded from further analysis30. These include a subset of non-learner mice (6/54 mice, 11.1%) that from the beginning of training miss the target aiming too far from the pellet or perform the grasping movement before they are in the correct position over the pellet. During the first training days, another group performed the task with high accuracy but started performing poorly by aiming too far from the pellet by day 3-4 (10/54 mice, 18.51%). Within this group, some mice start training using a preferred paw but change their preference after a few days; this has been previously discussed by Chen et al., 201430.
The reach-to-grasp movement is highly stereotypical from trial to trial and within animals (Figure 2). The use of high-speed videography allows tracking the trajectory of movements making it possible to analyze kinematics at different phases in the control condition and during optogenetic stimulation (Figure 1E and Figure 2C). This approximation results in a quantifiable assessment of parameters such as distance traveled, velocity, acceleration, end-point, and trajectory (Figure 2 C-E). It is possible to analyze both multi-reach trials, where the mouse reaches multiple times before retrieving the pellet, and single-trial events, where the mouse retrieves the pellet in a single reaching movement. The trial is finished when animals push the pellet away or reinitiate the trial by going to the rear of the cage. A quantitative comparison of trajectories under different experimental conditions is achieved with principal component analysis (PCA) followed by k-means clustering (Figure 3J-K)23,25.
During most training sessions, mice sometimes fail to grasp the pellet (missed trials). Some manipulations change the number of missed trials and hence the accuracy of the task. Then, it is essential to analyze differences between hit and missed trials. In our hands, missed trials result from changes in three distinct phases of the movement: (1) the paw modifies its trajectory before it crosses the chamber opening (initial error), (2) the paw modifies its trajectory after the paw crosses the opening (final error), and, (3) failure to collect the pellet (grasp error) (Figure 2 I,J)13. A general characteristic of missed trials is that mice start the grasping movement further away from the pellet (end-point) compared to hit trials (Figure 2G). Additionally, misses associated with the mouse posture are measured as significant differences in body angle between hit and missed trials (Figure 2H).
Depending on the structure or neuronal population targeted with optogenetics, one can expect differential effects over behavior7,19,23,31,32,33. The current protocol describes the impact of activating spiny projection neurons (SPNs) in the striatum in contralateral or ipsilateral hemispheres about the preferred paw used by the mouse during the reaching movement (Figure 3). Contralateral activation of D1 dopamine expressing SPNs, which give origin to the basal ganglia direct pathway, reduced grasping success to 64.9±8.8% compared to control conditions (Figure 3B). Kinematic analysis reveals that during optogenetic stimulation, the paw trajectory described an oscillatory pattern, shown by an increase in the traveled distance to 218.4±19.2% of control, leading to the incapability to target the pellet and an increase in initial error type I (Figure 3F). PCA analysis shows that all trials' trajectories during contralateral D1 SPNs activation separated in a cluster with almost no overlap with a control cluster, indicating a low similarity (Figure 3J-K).
On the other hand, activation of dSPNs in the ipsilateral side lead to an increase in trajectory dispersion shown by PCA analysis (Figure 3K) without affecting reaching success (120.7±23.6%, n = 4), total distance traveled (136.3±35.5%), or maximum velocity during the reaching phase (117.3±10.3%) (Figure 3C), indicating that ipsilateral D1 SPNs activation modified in some degree the reaching trajectory without changing the behavioral outcome (Figure 3G-I). Kinematic analysis indicates subtle changes in movement control by ipsilateral manipulation. Finally, body posture analysis shows a shift in body angle during contralateral D1SPNs activation (Figure 3L). It is highlighted that even this simple task has many components that permit proper movement execution to attain a goal.
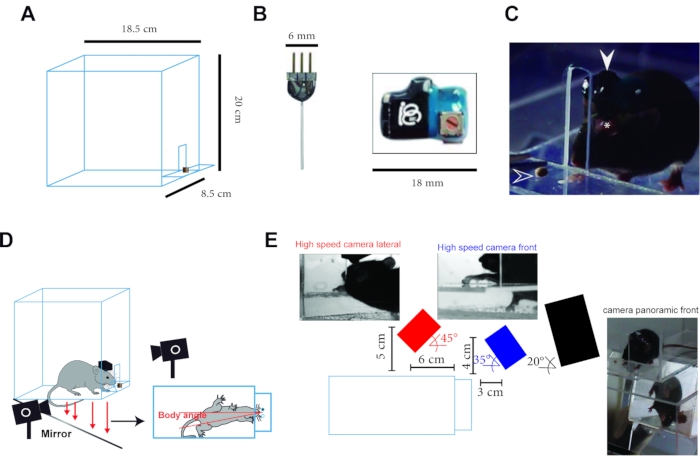
Figure 1: Experimental setup. (A) Schematics of the behavioral chamber. A chamber made with acrylic sheet having the following dimensions in cm: 18.5 (h) x 8.5 (w) x 20 (d) with a front window 1 (w) x 5 (h) and a small shelf 8.5 (w) x 4 (d). (B) Photograph of LED cannula (left) and wireless receiver (right). (C) Side view of a mouse with the implanted LED cannula connected to the receiver while performing the reach-to-grasp task (the white arrowhead shows the receiver, the asterisk shows the dental cement holding the cannula, and the empty arrowhead shows the pellet). (D) Sketch of the experimental setup. Two high-speed cameras record the reach in two dimensions, while a third collected a panoramic view of the task, including the mouse's location from the mirror under the chamber. Animals were free to choose their preferred paw, and stimulation sides always refer to the side of the preferred paw. (E) The exact position of the cameras and representative image of each camera during a trial. This figure has been adapted from Reference23. Please click here to view a larger version of this figure.
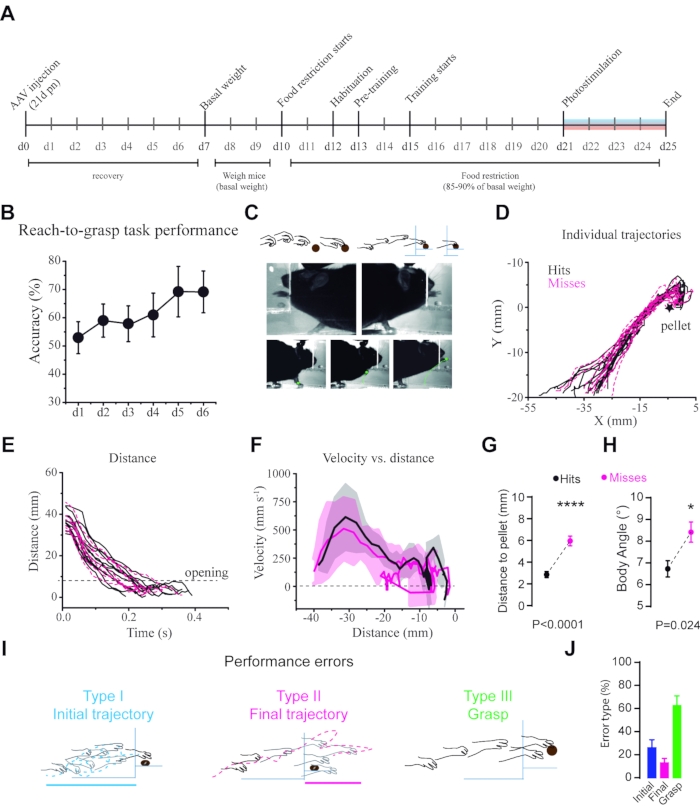
Figure 2: Kinematic analysis of reaching behavior. (A) Timeline of the experiment from day-0 (d0) to day-25 (d25). (B) Performance during the reach-to-grasp task over time measured as pellet retrieval accuracy (total number of successful grasps/total number of trials x 100). (C) Example of trajectory tracking from a high-speed video. (D) Individual trajectories of the paw during the hit and missed trials. (E) Total distance traveled by the paw during the hit and missed trials. (F) Acceleration of the paw through the trajectory in the hit and missed trials plotted as distance vs. velocity. (G) Summary of end-points distance in hits = 3.16 mm, misses 6.08 mm (Mann-Whitney-Wilcoxon testmissed vs. hit U = 4184, p<0.0001, n = 28 mice). (H) Differences in body angle in the two kinds of trials, misses = 8.4±5.3°, hits 6.7±4° (Mann-Whitney-Wilcoxon test U = 6437, P = 0.0243, n = 28 mice). (I) Schematics of the three types of errors. (J) Proportions of the three kinds of errors made by the mice in control conditions. This figure has been adapted from Reference23. Please click here to view a larger version of this figure.
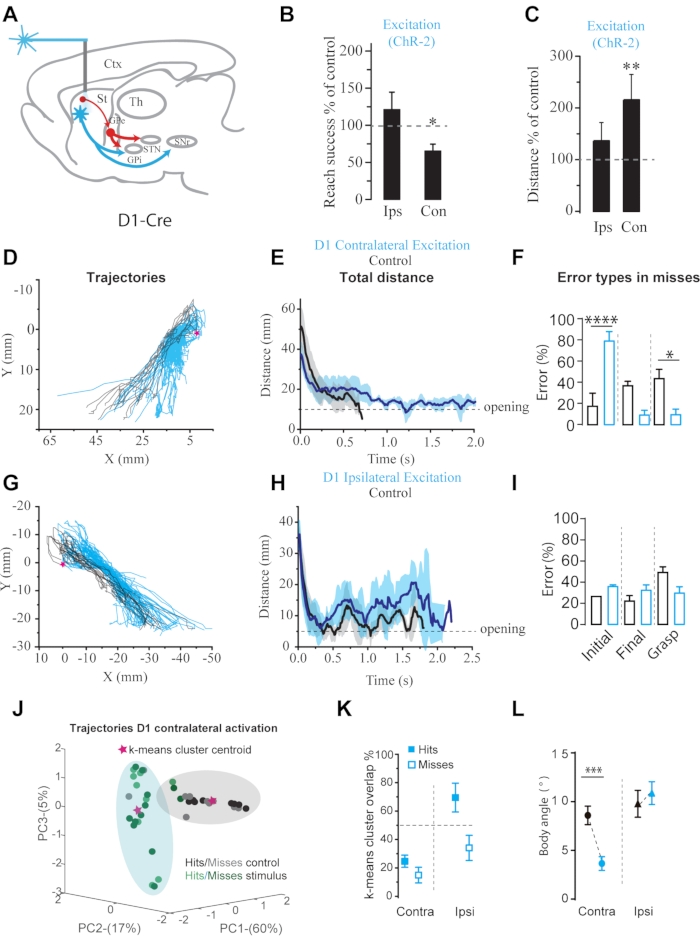
Figure 3: Optogenetic activation of contralateral and ipsilateral D1 SPNs during reach-to-grasp behavior. (A) Schematics of the stimulation paradigm. (B) Success rate compared to non-stimulation trials. (C) Change in traveled distance compared to control conditions. (D), (G) Two-dimensional plots of paths made by the paw with and without optogenetic stimulation. (E), (H) Total distance traveled by the paw during reaching movements. (F), (I) Summary of the distributions of the different kinds of errors. D1 contralateral: Initial error or type I (control = 18.2±11.6 %, stimulation = 79.9±8.2 % Fisher's exact test, p<0.0001). (J) Example of PCA analysis of the trajectories in control conditions compared to contralateral activation of D1SPNs. The shaded area represents the cluster of each condition, and the star is the cluster centroid. (K) Summary of the overlap between clusters of the different experimental conditions. (L) Changes in body angle. This figure has been modified from Reference23. Please click here to view a larger version of this figure.
Discussion
The use of optogenetic manipulation of neuronal populations in well-defined behavioral paradigms is advancing our knowledge about the mechanisms underlying motor control7,23. Wireless methods are especially suitable for tasks that require tests on multiple animals or free movement34,35. Nevertheless, as techniques and devices are refined, it should be the go-to option for any behavioral task combined with optogenetics34,36.
The current method has many advantages because miniaturized LEDs provide a reliable light source with high intensity, and implants can be used in studies requiring stimulation over several days. Nevertheless, insertion of an optic fiber for opsin stimulation may mechanically damage brain tissue, cause infections and occasionally inflammation at the location of the canula37. Long-lasting high-frequency optogenetic stimulation has been demonstrated to produce heat and could cause phototoxity37. It is possible to reduce phototoxicity by using red-shifted effector opsins that are activated with red or even near-infrared light, which reduces the generation of heat38.
Also, since the pins to connect the receiver remain outside of the skull, sometimes mice can cause displacement or detachment of the cannula if dental cement is not applied correctly; this often leads to damage of brain tissue and decreases the number of subjects to be taken into account for further analysis. Recent developments have introduced fiberless optogenetics, which uses particles that can emit visible light through up-conversion luminescence in response to near-infrared light penetrating deep in the brain tissue36. Fiberless devices bring the opportunity to stimulate optogenetically over longer time frames in freely behaving animals with unnoticeable implants35. This allows for unrestrained motion even in water mazes, to have multiple animals housed together (to avoid the impact of social isolation), and to study animals in more naturalistic settings35,36.
Even with all the advantages that fiberless optogenetics offers, it still faces biocompatibility and heat generation challenges. The efficiency of photon conversion also limits it. Finally, further improvement is required for high emission efficiency34,36.
The combination of this paradigm with high-speed videography allows for kinematic analysis under different experimental conditions. This offers sensitive detection of even subtle effects over distinct components of behavior and motor control. As more analytic tools develop, it is possible to have online kinematic analysis and an in-depth characterization of motor behavior in different contexts. A thorough quantification of mouse reaching movement kinematics has been recently published by Becker et al.25.
The possibility of selectively manipulating neuronal populations in freely moving animals with minimally invasive techniques allows one to dissect the contribution of specific neuronal types in precise behavioral tasks23. The reach-to-grasp task is a translatable paradigm for motor behavior13,19. It is known that conserved brain structures participate in the different phases of acquisition, learning, and performance of the task7,12,23. Revealing the neural circuits that underlie this behavior will increase the understanding of motor control. Several studies highlight the importance of bi-hemispheric control over unimanual tasks, especially when high dexterity is needed20,21,22. The kinematic analysis combined with optogenetic manipulations allows for the investigation of the different mechanisms of this complex behavior. It could help to analyze the contribution of sensory-motor feedback in normal conditions and disease models.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the UNAM-PAPIIT project IA203520. We thank the IFC animal facility for their help with mouse colonies maintenance and the computational unit for IT support, especially to Francisco Perez-Eugenio.
Materials
| Anaesthesia machine | RWD | R583S | Isoflurane vaporizer |
| Anesket | PiSA | Ketamine | |
| Breadboard | Thorlabs | MB3090/M | Solid aluminum optical breadboard |
| Camera lense | Canon | 50mmf/ 1.4 manual focus lenses (c-mount) | |
| Camera system | BrainVision | MiCAM02 | Camera controller and synchronizer |
| Cotton swabs | |||
| CS solution | PiSA | Sodium chloride solution 9% | |
| Customized training chamber | In house | ||
| Drill bit #105 | Dremel | 2 615 010 5AE | Engraving cutter |
| Dustless precission chocolate pellets | Bio-Serv | F05301 | |
| Ethyl Alcohol | J.T. Baker | 9000-02 | Ethanol |
| Eyespears | Ultracell | 40400-8 | Eyespears of absorbent PVA material |
| Fluriso | VetOne | V1 502017-250 | Isoflurane |
| Glass capillaries | Drumond Scientific | 3-000-203-G/X | Pipettes for NanoJect II |
| Hidrogen peroxide | Farmacom | Antiseptic | |
| High-speed camera | BrainVision | MiCAM02-CMOS | Monochrome high-speed cameras |
| Infrared emmiter | Teleopto | ||
| Insulin syringe | |||
| LED cannula | Teleopto | TelC-c-l-d | LED cannula 250um 487nm light |
| Micropipette 10 uL | Eppendorf | Z740436 | |
| Micro-pipette puller | Sutter | P-87 | Horizontal puller |
| Microscope LSM780 | Zeiss | Confocal microscope | |
| Microtome | |||
| Mock receiver | Teleopto | ||
| NanoJect II | Drumond Scientific | 3-000-204 | Micro injector |
| Oxygen tank | Infra | na | |
| pAAV-EF1a-double.floxed-hChR2(H134R)-mCherry-WPRE- HGHpA | Addgene | 20297 | Viral vector for ChR-2 expression |
| Parafilm | |||
| Paraformaldehyde | Sigma | P-6148 | |
| Phosphate saline buffer | Sigma | P-4417 | Phosphate saline buffer tablets |
| Pipette tips 10 uL | ThermoFisher | AM12635 | 0.5-10 uL volume |
| Pisabental | PiSA | Sodium pentobarbital | |
| Plexiglass | commercial | Acrylic sheet | |
| Povidone iodine | Farmacom | Antiseptic | |
| Procin | PiSA | Xylacine | |
| Puralube | Perrigo pharma | 1228112 | Eye lubricant 15% mineral oil/85% petrolatum |
| Rotary tool | Kmoon | Mini grinder | Standard |
| Scalpel | |||
| Scalpel blade | |||
| Stereotaxic apparatus | Stoelting | 51730D | Digital apparatus |
| Super-Bond C&B | Sun Medical | Dental cement | |
| Surgical dispossable cap | |||
| Teleopto remote controller | Teleopto | ||
| Tg Drd1-Cre mouse line | Gensat | 036916-UCD | Transgene insertion FK150Gsat |
| Tissue adhesive | 3M Vetbond | 1469SB | |
| TPI Vibratome 1000 plus | Peico | Microtome | |
| Vectashield mounting media with DAPI | Vector laboratories | H-1200 | Mounting media |
| Wireless receiver | Teleopto | TELER-1-P |
References
- Balbinot, G., et al. Post-stroke kinematic analysis in rats reveals similar reaching abnormalities as humans. Scientific Report. 8 (1), 8738 (2018).
- Klein, A., Sacrey, L. A., Whishaw, I. Q., Dunnett, S. B. The use of rodent skilled reaching as a translational model for investigating brain damage and disease. Neuroscience & Biobehavioral Reviews. 36 (3), 1030-1042 (2012).
- MacLellan, C. L., Gyawali, S., Colbourne, F. Skilled reaching impairments follow intrastriatal hemorrhagic stroke in rats. Behavioural Brain Research. 175 (1), 82-89 (2006).
- Evenden, J. L., Robbins, T. W. Effects of unilateral 6-hydroxydopamine lesions of the caudate-putamen on skilled forepaw use in the rat. Behavioural Brain Research. 14 (1), 61-68 (1984).
- Rodgers, R. A., Travers, B. G., Mason, A. H. Bimanual reach to grasp movements in youth with and without autism spectrum disorder. Frontiers in Psychology. 9, 2720 (2019).
- Sacrey, L. A. -. O., Zwaigenbaum, L., Bryson, S., Brian, J., Smith, I. M. The reach-to-grasp movement in infants later diagnosed with autism spectrum disorder: a high-risk sibling cohort study. Journal of Neurodevelopmental Disorders. 10 (1), 41 (2018).
- Azim, E., Jiang, J., Alstermark, B., Jessell, T. M. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 508 (7496), 357-363 (2014).
- Marques, J. M., Olsson, I. A. Performance of juvenile mice in a reach-to-grasp task. Journal of Neuroscience Methods. 193 (1), 82-85 (2010).
- Miklyaeva, E. I., Castaneda, E., Whishaw, I. Q. Skilled reaching deficits in unilateral dopamine-depleted rats: Impairments in movement and posture and compensatory adjustments. The Journal of Neuroscience. 14 (11), 7148-7158 (1994).
- Vaidya, M., Kording, K., Saleh, M., Takahashi, K., Hatsopoulos, N. G. Neural coordination during reach-to-grasp. Journal of Neurophysiology. 114 (3), 1827-1836 (2015).
- Wang, X., et al. Deconstruction of corticospinal circuits for goal-directed motor skills. Cell. 171 (2), 440-455 (2017).
- Xu, T., et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 462 (7275), 915-919 (2009).
- Ian, Q. W., Sergio, M. P. The structure of skilled forelimb reaching in the rat: A proximally driven movement with a single distal rotatory component. Behavioural Brain Research. 41 (1), 49-59 (1990).
- Proske, U., Gandevia, S. C. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiological Reviews. 92 (4), 1651-1697 (2012).
- Yttri, E. A., Dudman, J. T. Opponent and bidirectional control of movement velocity in the basal ganglia. Nature. 533 (7603), 402-406 (2016).
- Donchin, O., Gribova, A., Steinberg, O., Bergman, H., Vaadia, E. Primary motor cortex is involved in bimanual coordination. Nature. 395 (6699), 274-278 (1998).
- Brus-Ramer, M., Carmel, J. B., Martin, J. H. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. The Journal of Neuroscience. 29 (19), 6196-6206 (2009).
- d’Avella, A., Saltiel, P., Bizzi, E. Combinations of muscle synergies in the construction of a natural motor behavior. Nature Neuroscience. 6 (3), 300-308 (2003).
- Fattori, P., et al. Hand orientation during reach-to-grasp movements modulates neuronal activity in the medial posterior parietal area V6A. The Journal of Neuroscience. 29 (6), 1928-1936 (2009).
- vanden Berg, F. E., Swinnen, S. P., Wenderoth, N. Excitability of the motor cortex ipsilateral to the moving body side depends on spatio-temporal task complexity and hemispheric specialization. PLoS One. 6 (3), 17742 (2011).
- vanden Berg, F. E., Swinnen, S. P., Wenderoth, N. Involvement of the primary motor cortex in controlling movements executed with the ipsilateral hand differs between left- and right-handers. Journal of Cognitive Neuroscience. 23 (11), 3456-3469 (2011).
- Verstynen, T., Diedrichsen, J., Albert, N., Aparicio, P., Ivry, R. B. Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. Journal of Neurophysiology. 93 (3), 1209-1222 (2005).
- Lopez-Huerta, V. G., et al. Striatal bilateral control of skilled forelimb movement. Cell Reports. 34 (3), 108651 (2021).
- Lopez-Huerta, V. G., et al. The neostriatum: two entities, one structure. Brain Structure and Function. 221 (3), 1737-1749 (2016).
- Becker, M. I., Calame, D. J., Wrobel, J., Person, A. L. Online control of reach accuracy in mice. Journal of Neurophysiology. 124 (6), 1637-1655 (2020).
- Jaidar, O., et al. Synchronized activation of striatal direct and indirect pathways underlies the behavior in unilateral dopamine-depleted mice. European Journal of Neuroscience. 49 (11), 1512-1528 (2019).
- Gong, S., et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. The Journal of Neuroscience. 27 (37), 9817-9823 (2007).
- Rowland, N. E. Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comparative Medicine. 57 (2), 149-160 (2007).
- Ullman-Culleré, M. H., Foltz, C. J. Body condition scoring: a rapid and accurate method for assessing health status in mice. Laboratory Animal Science. 49 (3), 319-323 (1999).
- Chen, C. C., Gilmore, A., Zuo, Y. Study motor skill learning by single-pellet reaching tasks in mice. Journal of Visualized Experiments. (85), e51238 (2014).
- Fink, A. J., et al. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature. 509 (7498), 43-48 (2014).
- Li, Q., et al. Refinement of learned skilled movement representation in motor cortex deep output layer. Nature Communication. 8, 15834 (2017).
- Overduin, S. A., d’Avella, A., Carmena, J. M., Bizzi, E. Microstimulation activates a handful of muscle synergies. Neuron. 76 (6), 1071-1077 (2012).
- Miyazaki, T., et al. Large Timescale interrogation of neuronal function by fiberless optogenetics using lanthanide micro-particles. Cell Reports. 26 (4), 1033-1043 (2019).
- Yang, Y., et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nature Neuroscience. 24 (7), 1035-1045 (2021).
- Kampasi, K., et al. Fiberless multicolor neural optoelectrode for in vivo circuit analysis. Scientific Reports. 6, 30961 (2016).
- Allen, B. D., Singer, A. C., Boyden, E. S. Principles of designing interpretable optogenetic behavior experiments. Learning & Memory. 22 (4), 232-238 (2015).
- Packer, A. M., et al. . Nature Methods. 9, 1202-1205 (2012).

