免疫組織化学と免疫細胞化学:光顕微鏡による組織イメージング
English
Share
Overview
ソース: マイケル S. リー1とトーニャ J. ウェッブ1
1メリーランド大学医学部微生物学・免疫学科、マーリーン・スチュワート・グリーンバウム総合癌センター、ボルチモア、メリーランド州 21201
免疫組織化学(IHC)および免疫細胞化学(ICC)は、抗体を用いた特定抗原の発現および局在化を可視化するために用いられる技術である。IHCの最初の使用は、アルバート・クーンズが肺炎球菌に感染したマウスからの組織切片における肺炎球菌抗原の存在を視覚化する技術を使用した1941年でした(1)。免疫組織化学という名前は、IHCで使用される組織切片を参照して、抗体を参照して「免疫-」、および「histo-」の根に由来する。免疫細胞化学の根元「サイト-」は、ICCとIHCの主な違いを強調しています。IHCは組織全体の切片を使用しますが、ICCは組織から単離された細胞や培養中に増殖した細胞を使用します。使用されるサンプルの違いは、サンプル調製が技術的にIHCとICCの間で異なることを意味しますが、そうでなければICCとIHCのプロトコルは同一であり、用語は頻繁に同じ使用されることがわかります。
IHCおよびICCの両方において、ペルオキシダーゼまたはロダミンなどの化学的または蛍光タグを有する抗体は、タグ付けされた抗体を抗原に対する特異的結合を通じて目的の任意の抗原の分布を可視化するために使用される。IHCの場合、組織の薄いスライスは、染色される前に組織の構造を維持するためにスライド上に固定され、組織全体のコンテキストで抗原の視覚化を可能にする(図1)。ICCの場合、細胞は染色される前にスライド上に均等に分布し、個々の細胞内の抗原分布の可視化を可能にするが、特定の組織の構造内には存在しない。2 つのプロトコル間の類似性により、このプロトコルは IHC に関連するサンプル調製の追加の複雑さに対処するために IHC に焦点を当てます。
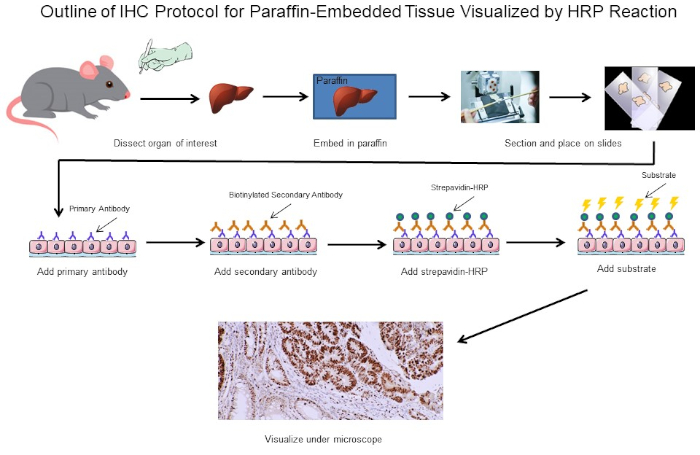
図1:IHCプロトコルの概要マウスから解剖したパラフィン埋め込み組織のIHCプロトコルの視覚的輪郭。このプロトコルは、ビオチン化二次抗体とストレパビジン-HRPを使用して、抗体結合の位置を可視化します。蛍光タグ付き抗体などの他のオプションも可能です。この図のより大きなバージョンを表示するには、ここをクリックしてください。
IHCを行う際の最初の主要な決定は、染色プロセス全体を通して組織の構造を維持するために組織セクションを調作成する方法である。2つの主要な選択は、パラフィン埋め込み組織のホルマリン固定セクションまたは凍結組織の新鮮なセクションである。どの方法を使用するかは、下流の解析を行うかによって異なります。パラフィン埋め込み組織のホルマリン固定は、一般的に、新鮮な組織を凍結しながら、IHCの外の後続のアッセイのためのタンパク質機能を維持しながら、最適なイメージングのための組織形態をより良く保存すると考えられている。さらに、新鮮な凍結組織切片は、遺伝子発現分析(2)により適することが示されている。第3の考慮事項は、一部の抗体は特定のタイプのセクションに対してのみ最適化され、他の抗体では機能しない可能性があるため、目的の抗原に対する抗体が固定組織セクションまたは凍結組織切片に適しているかどうかです。最後に、新鮮な凍結サンプルは-80°Cで保存する必要があり、固定されたセクションは室温ではるかに長く保存することができる間、1年を超えて持続しない可能性があるため、組織切片を保存する必要がある期間を決定する必要があります。これらは、パラフィン埋め込み組織のホルマリン固定切片または凍結組織の新鮮なセクションを使用するかどうかを決定するための主要な考慮事項のいくつかです。最終的には、十分な組織を持っている場合は、両方の一部を持っているだけで良いかもしれません。
本実験では、リンパ腫発症の自発的マウスモデルから拡大脾臓においてサイクリンD1発現が増加したかどうかを調べる。脾臓組織試料は、まず、野生型マウス、リンパ腫を有しないトランスジェニックマウス、または自発的にリンパ腫を発症したトランスジェニックマウスのいずれかから単離した。脾臓組織試料をパラホルムアルデヒドに固定し、パラフィンに埋め込み、切除し、マウス抗サイクリンD1一次抗体を用いて染色し、続いて馬の抗マウス二次抗体を用いて、3,3-ジアミノベンジジン(DAB)を用いて開発した。その後、ハリス・ヘマトキシリン溶液でセクションを逆染色し、その後、セクションを20倍の倍率で画像化した。
試薬
パラフィン埋め込みセクション
- 4% パラホルムアルデヒド(PFA)
- エタノール(無水変性、組織学的等級100%、95%、80%、75%、50%)。二重蒸留水(ddH2O)を使用して100%のストックから希釈することができます
- キシレン
- IHC互換ガラススライドは、組織セクションが手順全体を通して取り付けられたままであることを確認します。IHC互換ガラススライドは、特殊なコーティングを持っており、複数の小売業者から容易に入手可能です。ICCを実行する場合は、チャンバスライドを使用してください。チャンバースライドは、細胞がチャンバーに播種され、細胞がスライドに付着し、適切な合流に達するまでインキュベーターに置くことができ、その時点でチャンバーを除去し、染色をIHCと同様に進めることができます。
- パラフィン
- 0.3% 過酸化水素 (H2O2)/メタノール: 調味するには、1 mL 30% H2O2 ~ 99 mL メタノールを添加します。-20°Cでの保管
- 抗原検索バッファー: IHC クレートバッファー pH 6.0
新鮮な冷凍セクション
- 最適な切断温度(OCT)埋め込み化合物
- 最適な固定性:-20°Cに冷却された4%のPFAまたはアセトン
染色
- ブロック バッファ: ユーザーが決定する必要があります。一例は、1X PBSで希釈された馬血清です
- 希釈された一次抗体:メーカー仕様を参照
- 希釈ビオチン化二次抗体:メーカー仕様参照
- 希釈されたアビジン-ホースラディッシュペルオキシダーゼ(HRP):ペルオキシダーゼ可視化のみ。製造元の仕様を参照してください。
- DABまたは他の互換性のある基板
- カウンターステイン(オプション)
- エタノール(無水変性、組織学的グレード100%および95%)
- キシレン
- オルガノ/リモネン山
Procedure
Results
IHC and ICC have a vast range of applications. For example, one use of IHC is to examine the expression of oncogenes in spontaneous mouse models of tumor development. In Figure 2, we set out to determine if cyclin D1 expression was increased in enlarged spleens in a spontaneous mouse model of lymphoma development. Splenic tissue samples were fixed in paraformaldehyde, embedded in paraffin, sectioned, stained using an anti-cyclin D1 antibody (diluted 1:200 in blocking buffer), and then the sections were imaged at 20X magnification. Cyclin D1 expressing cells are indicated by the reddish-brown color against the blue tissue background. These results suggest that cyclin D1 expression was increased in enlarged spleens, indicating a correlation between cancer development and cyclin D1 expression in this model.
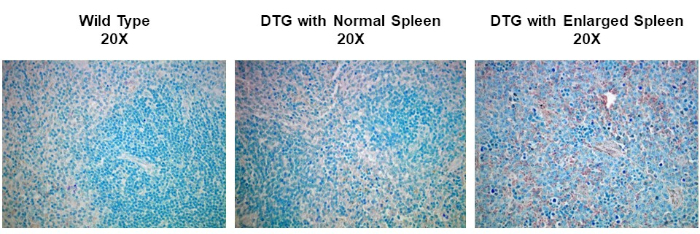
Figure 2: Splenic Cyclin D1 Expression in a Spontaneous Double Transgenic (DTG) Mouse Model of Lymphoma. An image of splenic tissue stained with an anti-Cyclin D1 primary antibody, counterstained with methyl green, and visualized using a biotinylated secondary antibody and ABC reagent activated with DAB substrate. The reddish-brown color represents locations where the antibody has bound indicating the presence of Cyclin D1 expressing tumor cells within the structure of splenic tissue that has been counterstained blue. Please click here to view a larger version of this figure.
Applications and Summary
Immunohistochemistry (IHC) and immunocytochemistry (ICC) are techniques used to visualize the expression and localization of specific antigens using antibodies. Tissues are first cut into thin sections that maintain the tissue morphology and placed on a slide. The antibodies are then added and will bind the antigen of interest and are equipped with a specific tag that allows them to be visualized under a microscope. Thus, through this basic concept, the distribution of antigens in the context of tissue structure can be visualized and studied. However, while the overarching concept is basic, there are multiple different approaches and variations that have been developed that increase both the complexity and usefulness of these techniques. This paper has covered the basic concept of IHC and ICC, the main decisions that need to be considered when using these techniques, and a detailed step-by-step protocol. The images produced by IHC and ICC are generally the final product and can be published as is to highlight obvious differences in amounts or distribution of staining between different conditions.
References
- Coons, A. H. Creech, H. J., Jones, N. and Berliner, E. The Demonstration of Pneumococcal Antigen in Tissues by the Use of Fluorescent Antibody, The Journal of Immunology, 45 (3), 159-170 (1942).
- Ripoli, F. L., Mohr, A., Hammer, S. C., Willenbrock, S., Hewicker-Trautwein, M., Hennecke, S., Escobar, H. M. and Nolte, I. A comparison of fresh frozen vs. Formalin-fixed, paraffin-embedded specimens of canine mammary tumors via branched-DNA assay. International Journal of Molecular Sciences, 17 (5) (2016).
Transcript
Immunocytochemistry and immunohistochemistry are staining methods for a protein of interest in cultured cells and tissues, respectively. The basic principle of both related techniques involves using specific antibodies tagged with a detection system to identify and visualize the protein and determine its location within the cells and tissues, as well as the relative levels. The process in either experiment begins with sample preparation.
For immunocyctochemistry, which specifically visualizes protein or antigen locations in cells, this involves three steps. The first step is plating, which entails culturing the cells in growth media on a cover slip or slide, typically, in the wells of a culture plate. This is followed by fixation, where a precipitating or crosslinking agent like paraformaldehyde is added to the cells to preserve the structural integrity of the proteins and prevent enzyme activity from degrading them. The last step is permeabilization, which involves adding a detergent to make the cell membranes permeable for the staining.
In the counterpart method, immunohistochemistry, proteins or antigens are visualized in tissues and sample preparation has five steps. First, the whole tissue is subjected to fixation, usually with paraformaldehyde. This is followed by embedding of the tissue in a block of paraffin, and then sectioning of this block using a machine called a microtome to cut the tissue into thin slices which can be placed onto slides. Next, the slides are subjected to deparaffinization, or removal of the paraffin from around the tissue slice. Then, an optional antigen retrieval step can be performed. This can either be done using heat or enzymes to unmask epitopes that were cross-linked during fixation making them available for antibody binding. After the appropriate sample preparation, a target-specific primary antibody is added to the cell or tissue sample. This primary antibody should bind to the protein of interest. Next, a secondary antibody is added, which detects and binds to the primary antibody. This secondary antibody is conjugated to, or can bind to, an enzyme called HRP. When its specific substrate, DAB, is added, HRP converts this to an insoluble, brown precipitate. This brown stain marks the location of the target protein. The slides are also stained with hematoxylin, which labels the nuclei in blue and provides a spatial reference point for determining subcellular localization. After that, mounting media is added to the slide, followed by a cover slip in order to seal and preserve the stained sample. Finally, the slides can be imaged on a light microscope.
In this video, you will observe the sample preparation technique for plated cells and tissue sections, followed by immunostaining of the tissue sections.
First, the cells of interest need to be seated onto coverslips. To do this, working in a tissue culture hood, place individual coverslips into the wells of a 24-well plate. Then, close the sash and turn on the UV light to sterilize the coverslips for at least 15 minutes. Next, turn off the UV light. To lift the cells of interest from a confluent 10-centimeter dish, aspirate the media, wash briefly with PBS, and add trypsin to the cells for 2 minutes. Then, tap the side of the plate to ensure the cells have detached and neutralize the trypsin with media. Next, add 0. 5 mL of the cell suspension into each well, making sure to cover the coverslips. Place the plate into a humidified CO2 incubator and allow the cells to grow at 37 degrees celsius until they are 50-70% confluent.
Once the cells reach the optimal confluency, aspirate the culture medium from each well, and then fix the cells by incubating them in . 5 mL of 4% paraformaldehyde diluted in 1X PBS for 20 minutes at room temperature. After removing the fixative, rinse the cells be adding 1 mL of 1X PBS over each coverslip. Immediately aspirate the PBS, then repeat the rinse 2 more times for a total of 3 washes.
Now, permeablize the cells by adding 0.5 mL of 0.1% Triton X-100 in 1X PBS to each well. Leave the plate at room temperature for 15 minutes. Aspirate off the permeabilization buffer and then rinse the cells by adding 1 mL of 1X PBS into each well. Immediately aspirate off the PBS and repeat the rinse 2 more times for a total of 3 washes. Now that the cells on the coverslips are fixed and permeabilized, proceed to the staining procedure demonstrated for the following immunohistochemistry example with the exception that the incubations should be performed within the wells of the 24-well plate rather than directly on a tissue section slide.
To begin, obtain prepared, formalin-fixed, paraffin-embedded tissue sections. Deparaffinize the slides by placing them into a slide rack and then completely immersing them into 250 mL of 100% xylene. Allow the slides to incubate for 5 minutes in the xylene. Then, remove the slides from the container, wipe them off with a paper towel, and place them into a new xylene bath in a fresh container for a further 5 minutes.
Next, rehydrate the sections in a series of graded ethanol solutions starting with 100% ethanol for 3 minutes. Wipe off the slide rack with a paper towel and transfer the slides to a new container of 100% ethanol for another 3 minutes. Continue this cycle of washing, drying with a paper towel, and transferring the slides to a new bath following the indicated concentrations of ethanol for the specified time. After the final ethanol wash, wipe off the rack with a paper towel and incubate the slides in 100 mL of .3% hydrogen peroxide for 30 minutes at room temperature in order to block any endogenous peroxidase activity. Wash the slides in 250 mL of 1X PBS for 5 minutes. Repeat this wash in a container of fresh 1X PBS for an additional 5 minutes.
Next, perform antigen retrieval by immersing the slides in 250 mL of IHC citrate buffer at pH 6.0 and boiling them for 20 minutes. Then, proceed to the staining protocol.
To begin the staining process for IHC, circle the sections with a hydrophobic pen to identify the minimal area that the buffer needs to cover. Then, use a pipette to place 100 microliters of blocking buffer, which in this experiment is horse serum diluted in 1X PBS, over the section. Incubate the slides for 1 hour at room temperature. Following this, remove the blocking buffer using a pipette.
Next, dilute the primary antibody and blocking buffer at a 1:100 dilution by adding 990 microliters of horse serum diluted in 1X PBS into a 1. 5 mL Eppendorf tube, followed by 10 microliters of the primary antibody. Add 100 microliters of the diluted primary antibody to each section, and incubate the slides for 30 minutes at room temperature. When the timer sounds, drain the primary antibody off each slide, and then wash them in 250 mL of 1X PBS for 5 minutes. Repeat this wash once more using fresh 1X PBS.
While the slides are washing in 1X PBS, dilute the secondary antibody to a 1:200 dilution by adding 995 microliters of blocking buffer to a 1.5 mL tube followed by 5 microliters of the secondary antibody, which in this case is biotinylated horse anti-mouse IGG. Add 100 microliters of the diluted secondary antibody to each section, and then incubate the slides for 30 minutes at room temperature. After 30 minutes, remove the secondary antibody by draining it off the sections, then wash the slides in 250 mL of 1X PBS for 5 minutes. Repeat this wash using fresh 1X PBS.
Now, add 100 microliters of avidin-biotin complex reagent, and incubate the sections in the dark for 30 minutes at room temperature. Next, wash the slides by immersing them in 250 mL of 1X PBS for 5 minutes. Similar to previous wash steps, repeat this wash one more time using fresh 1X PBS. Next, develop the slides by incubating the sections in 100 microliters of DAB for up to 5 minutes. Stop the development by immersing the sections in 250 mL of distilled water for 5 minutes.
Now, slides can be counterstained, if desired. To do this, briefly dip the slides in 250 mL of Harris Hematoxylin Solution. Rinse off the counterstain by washing the slides in 250 mL of distilled water for 5 minutes. Repeat this wash 1 more time using fresh distilled water. Next, dehydrate the sections. To do this, first incubate the slides in 95% ethanol for 5 minutes. Blot the slides on a paper towel, and transfer them to a new container of fresh 95% ethanol for another 5 minutes. Continue the cycle of washing, blotting with a paper towel, and transferring the slides to a new bath, following the indicated solutions for 5 minutes each.
After the final incubation, blot the slides with a paper towel, then add a drop of mounting media, such as Organo-Limonene Mount, to the slides. Now, place a coverslip over the sections, taking care not to trap air bubbles. The slides are now ready to be observed under a microscope for analysis.
To observe the stained sections, use a standard light microscope to visualize the stain, and a digital camera to capture the image. In this particular example of IHC, spleen tissues from wild type and spontaneous, double-transgenic, or DTG mice, are compared for studying Dyclin D1 expression in lymphoma. The tissues were paraffin-embedded, sectioned, and stained with anticyclin D1 antibody, and imaged at 20X magnification. Cyclin D1 expressing cells are indicated by the reddish-brown color against the blue tissue background. Comparing the staining intensities among the images from the various mice, the non-enlarged spleens have relatively low amounts of Cyclin D1 expression irrespective of the mouse genotype. In contrast, the enlarged spleen from the DTG mouse, shows increased reddish-brown staining indicating a correlation between cancer development and Cyclin D1 expression in this mouse model.
