Study Glial Cell Heterogeneity Influence on Axon Growth Using a New Coculture Method
Summary
In this protocol, we described a new method to study the influence of glial cell heterogeneity on axon growth with an in vitro co-culture system. Rat cortical glial cells were cultured to confluence and cocultured with highly purified rat dorsal root ganglia neurons. Different glial cell influence on neurons adhesion and axon growth was compared directly in the same culture. This method provides a new way to directly study the glial cell heterogeneity influence on neuron adhesion and axon growth.
Abstract
In the central nervous system of all mammals, severed axons after injury are unable to regenerate to their original targets and functional recovery is very poor 1. The failure of axon regeneration is a combined result of several factors including the hostile glial cell environment, inhibitory myelin related molecules and decreased intrinsic neuron regenerative capacity 2. Astrocytes are the most predominant glial cell type in central nervous system and play important role in axon functions under physiology and pathology conditions 3. Contrast to the homologous oligodendrocytes, astrocytes are a heterogeneous cell population composed by different astrocyte subpopulations with diverse morphologies and gene expression 4. The functional significance of this heterogeneity, such as their influences on axon growth, is largely unknown.
To study the glial cell, especially the function of astrocyte heterogeneity in neuron behavior, we established a new method by co-culturing high purified dorsal root ganglia neurons with glial cells obtained from the rat cortex. By this technique, we were able to directly compare neuron adhesion and axon growth on different astrocytes subpopulations under the same condition.
In this report, we give the detailed protocol of this method for astrocytes isolation and culture, dorsal root ganglia neurons isolation and purification, and the co-culture of DRG neurons with astrocytes. This method could also be extended to other brain regions to study cellular or regional specific interaction between neurons and glial cells.
Protocol
1. Glia Cell Culture
Glial cells can be cultured from different regions of central nervous system. The whole process is shown in process figure.
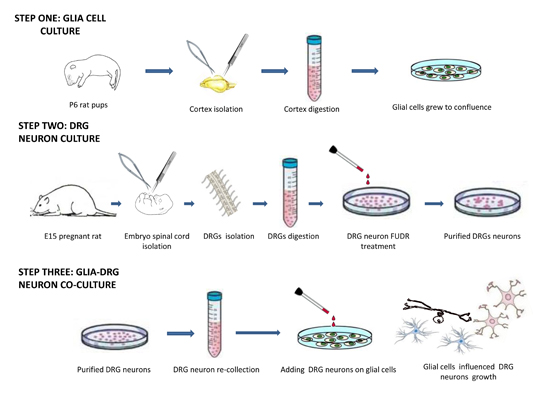
Day 1 Coating culture plate and coverslips
- Dry sterilized glass microscope round coverslips in an autoclave.
- Plate the sterilized coverslips into sterilized 24-well culture plate.
- Coat the coverslips with poly-lysine and incubate for 2 hours under root temperature.
- Coat 6-well culture plates in the same way as step 3.
- Wash the coverslips and 6-well culture plate twice with distilled water and air dry in culture hood.
- Add 200 μL DMEM medium(with 10% FBS) in each well in 24-well plate and 2 mL in each well in 6-well plate and put them into incubator under 37 degree with 5% CO2
Day 2 Isolating cortex and glial cell culture
- Sterilize the positive flow dissection hood.
- Turn on UV light for 20 min.
- Spray all surfaces with 70% ethanol and wait 15 min before use.
- change tense: Anesthetize rat pups (P2-P6) by hypothermia, and decapitate at the base of the framen magnum using operating scissors.
- Open the cranium along the sagittal suture using an iris scissor and peel off the skull.
- Remove the forebrain and put them into chilled L15 medium, under stereomicroscope, carefully clean the dura and pia membrane with blood vessels, isolate the cortex and wash several times with L15 medium.
- Cut the cortex into small pieces with microsurgery scissor.
- Add 0.125% trypsin-EDTA prepared with L15 medium into cortex pieces and incubate in 37 degree for 15 min.
- Transfer tissue blocks into 20mL DMEM medium with 20% FBS in 50mL tube, add DNase stock solution to final concentration 10ug/mL, aspirate the tissue solution up and down for 20 times with fire polished glass Pasteure pipette, collect the single cells suspension.
- Wash the cell suspension one time with DMEM medium, re-suspend cells in DMEM with 10% FBS, count cells under microscope with a hemocytometer, seed cells at 5000-10000/cm2. Maintain cells in a 37 degree 5% incubator, change half of the medium 2 times per week.
2. Dorsal Root Ganglia Neurons Isolation, Culture and Purification
Day 1 Prepare Culture Material
- Coat the sterilized coverslips with poly-lysine, wash coverslips twice with distill water and air dry, put them into 24-well plates.
- Add 100μL Neurobasal medium with 2% B27 and 2.5S NGF (50 ng/mL), put plate into 37 degree 5% CO2.
Day 2 Isolate DRGs from embryos
- Sterilized the flow hood in the same way as glial cell culture.
- Euthanize a pregnant rat (E15) by overdose with CO2, sterilized abdomen by 70% ethanol, embryos were isolated from uterus and put into chilled L15 medium.
- Isolate Spinal cords with connected dorsal root ganglia under stereomicroscope and transfer to 35mm dishes with chilled L15 medium.
- Collect single cell suspension and wash once with NBF medium (Neurobasal medium containing 2% B27 and 2.5S NGF (50 ng/mL)).
Day 3 Purify DRG Neurons
- 18h-24h after neuron seeding, add a 1 mM concentrated FUDR stock solution to the neuron culture to a final concentration of 20 μM and a final volume of 200 μL.
- 72h later, replace half the medium with Neurobasal medium containing 2% B27 and 2.5S NGF (50ng/mL) without FUDR. After that, change the medium every other day.
3. Coculture DRG neurons with glial cells
- When glial cell culture reached confluence (around 20 days after seeding), they were ready for coculture with neurons.
- 24 hours before adding the purified neurons, glial cells medium were changed to Neurobasal medium containing 2% B27 and 2.5S NGF(50 ng/mL).
- Purified neurons were collected from culture wells, single cells suspension were obtained by mechanical passing through fire polished Pasteure pipette.
- After counting the number, neurons were seeded at 500/cm2 on confluent glial cells in Neurobasal medium containing 2% B27 and 2.5S NGF (50ng/mL).
- Neurons adhesion and neurite growth on glial cells could be recorded and analyzed at different time points by image analysis software and immunocytochemistry using cell type specific antibodies.
4. Representative Results
- Glial cell culture: After seeding, glial cells will become confluent around 20 days. Under phase contrast microscope, it was easily identified that different morphological glial cell subpopulations formed different growth pattern substructures and the GFAP positive astrocytes accounted for more than 90% as shown by immuocytochemisty technique (Figure 1, Figure 2).
- DRG neurons culture: In our method, after 72 hours FUDR treatment, the purity of DRG neurons will reach as high as 99% within 6 days, DRG neurons showed their unique morphologies and with high density neurite growth(Figure 3, Figure 4).
- Glial cell and neurons coculture: DRG neurons adhesion and neurite outgrowth occurred readily on glial cells within 4 hours after seeding, careful observations showed that neuron adhesion and neurite growth were influenced by glial cell subpopulations which formed special growth pattern substructures, this could be easily identified under phase contrast microscope and by immunocytochemistry(Figure 5, Figure 6).
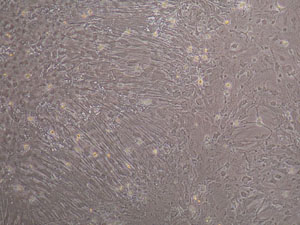
Figure 1. Morphology of confluent glial cells. Cortical glial cells were plated on polylysine coated coverslip and cultured for 20 days, note the different growth pattern of glial cells, cells on the left side arranged in a radiated way.

Figure 2. Confluent glial cells labeled by Glial fibrillary acidic protein (GFAP) antibody. Note the radiated arrangement of GFAP (red) positive cells on the right side.
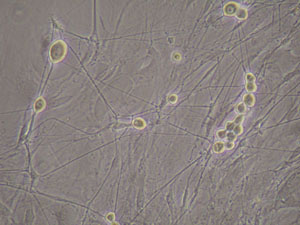
Figure 3. Dorsal root ganglia neurons grew in vitro without FUDR treatment. The contaminating cells formed DRG neurons’ background.
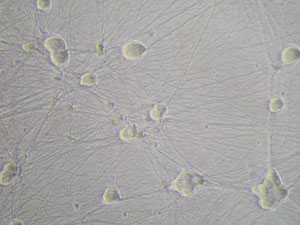
Figure 4. Dorsal root ganglia neurons grew in vitro after FUDR treatment. The background contaminating cells had been eliminated completely.
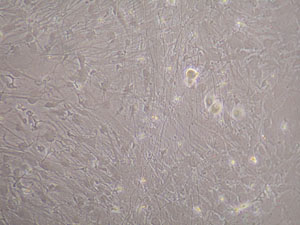
Figure 5. Dorsal root ganglia neurons grown on glial cells. Neurons adhesion and neurite growth were inhibited on the radiated arranged cells and limited on the right side glial cells.
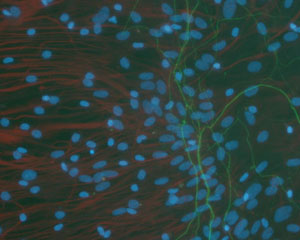
Figure 6. Dorsal root ganglia neurons growing on glial cells labeled by neurofilament antibody. The neurite(green) were inhibited on the radiated arranged left side glial cells and limited on the right side, all the glial cells were labeled by GFAP antibody(red).
Discussion
This experiment protocol was designed to reach two goals to study glial cells, especially astrocyte heterogeneity’s influence on neuron adhesion and neurite growth. The first goal was to maintain astrocyte heterogeneity as much as possible, in this experiment, the confluent glial cell culture enriched astrocytes was mixed primary culture without any chemical treatment and digestion propagation, which may further damage the cell and induce injury response of astrocytes. The final astrocyte purity is more than 90% GFAP positive, with contamination fibroblasts less than 1% as verified by fibrobasts FN. The low seeding density at beginning made cells undergo several rounds of division before confluence. Under phase contrast microscope, the glial cell heterogeneity was observed by the different substructure formed by different morphological astrocytes. The second goal was to get high purified DRG neurons and coculture them with glial cells to study the heterogeneity influence on neuron behavior. Contaminating cells in dorsal root ganglia such as fibroblasts and schwann cells could apparently influence or change the axon growth in culture conditions 5, 6, to avoid this unwanted influence, DRG neurons were cultured in serum free medium and treated by FUDR for 72h to kill all the other cell types. After this treatment, DRG neurons could be purified as high as 99%. It was more efficient than traditional method which needs to treat the neurons culture several times 7. Single DRG neuron adhesion and axon growth could be easily monitored and tracked under phase contrast microscope by time lapse recording and imaging analysis method, their interaction with glial cells could be analyzed in details by immunocytochemistry and electronic microscope technique.
It was noted that DRG neurons could not be maintained in vitro for more than 4 weeks, their survival had no sign of reduction but they could not adhere to the substrate any longer, usually they would detach and roll up.
DRG neurons could be maintained on astroglial cells for a long time (more than 2 months). This long term coculture between high purified DRG neurons and astrocytes make the tracking of single neurons and axon growth on different type of astrocytes more easily, it also avoid the uncontrolled influence from other contamination cells in dorsal root ganglia. During the whole culture process, all cells should be handled carefully and all the cells should always be immersed in culture medium. To get high purity DRG neurons, a critical point is the duration of FUDR treatment, more than 72h treatment will significantly compromise cellular viability and short time treatment may not kill all the contamination cells. In the optimal condition, we can obtain large amount of high purity DRG neurons within 1 week. Using this coculture system, we found that, in contrast to commonly held beliefs, heterogeneous astrocytes had different influences on neurons adhesion and axon growth, and subpopulation astrocytes showed strong inhibition to both neuron adhesion and axon growth. Besides the interaction between astrocyte and axon growth, this method could be used in on other studies such as myelin formation, neuronal development changes in intrinsic axon growth capacity, genes and signal pathways related to axon growth. It can also be used in mice and other animals’ central nervous system regions to study the interaction between neurons and glial cells.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by FMMU new finding foundation and partially NIH funding.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| DMEM(high glucose) | Invitrogen | 10313-039 | ||
| L15 medium | Invitrogen | 11415-114 | ||
| FBS | Invitrogen | 10437-077 | ||
| Neurobasal Medium | Invitrogen | 21103-049 | ||
| poly-lysine | Sigma | P4832 | culture grade | |
| NGF(2.5S) | Invitrogen | 13257-019 | ||
| B27 supplyment | Invitrogen | 17504-044 | ||
| 0.25% trypsin-EDTA | Invitrogen | 25200-056 | ||
| FUDR | Sigma | F0503 | ||
| neurofilament antibody | Abcam | ab24575 |
References
- Rossignol, S., Schwab, M., Schwartz, M., Fehlings, M. G. Spinal cord injury: time to move?. J Neurosci. 27 (44), 11782-11792 (2007).
- Yiu, G., He, Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 7 (8), 617-627 (2006).
- Barres, B. A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 60 (3), 430-440 (2008).
- Matyash, V., Kettenmann, H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. , (2009).
- Wanner, I. B., Deik, A., Torres, M., Rosendahl, A., Neary, J. T., Lemmon, V. P., Bixby, J. L. A new in vitro model of the glial scar inhibits axon growth. Glia. 56 (15), 1691-1709 (2008).
- Kuffler, D. P., Sosa, I. J., Reyes, O. Schwann cell chondroitin sulfate proteoglycan inhibits dorsal root ganglion neuron neurite outgrowth and substrate specificity via a soma and not a growth cone mechanism. J Neurosci Res. 87 (13), 2863-2871 (2009).
- Kleitman, N., Wood, P. M., Bunge, R. P. Tissue culture method for the study of myelination. Culturing nerve cells. , P559-P559 (1998).

