Development of a Backbone Cyclic Peptide Library as Potential Antiparasitic Therapeutics Using Microwave Irradiation
Summary
A simple and general method for the synthesis of cyclic peptides using microwave irradiation is outlined. This procedure enables the synthesis of backbone cyclic peptides with a collection of different conformations while retaining the side chains and the pharmacophoric moieties., and therefore, allows to screen for the bioactive conformation.
Abstract
Protein-protein interactions (PPIs) are intimately involved in almost all biological processes and are linked to many human diseases. Therefore, there is a major effort to target PPIs in basic research and in the pharmaceutical industry. Protein-protein interfaces are usually large, flat, and often lack pockets, complicating the discovery of small molecules that target such sites. Alternative targeting approaches using antibodies have limitations due to poor oral bioavailability, low cell-permeability, and production inefficiency.
Using peptides to target PPI interfaces has several advantages. Peptides have higher conformational flexibility, increased selectivity, and are generally inexpensive. However, peptides have their own limitations including poor stability and inefficiency crossing cell membranes. To overcome such limitations, peptide cyclization can be performed. Cyclization has been demonstrated to improve peptide selectivity, metabolic stability, and bioavailability. However, predicting the bioactive conformation of a cyclic peptide is not trivial. To overcome this challenge, one attractive approach it to screen a focused library to screen in which all backbone cyclic peptides have the same primary sequence, but differ in parameters that influence their conformation, such as ring size and position.
We describe a detailed protocol for synthesizing a library of backbone cyclic peptides targeting specific parasite PPIs. Using a rational design approach, we developed peptides derived from the scaffold protein Leishmania receptor for activated C-kinase (LACK). We hypothesized that sequences in LACK that are conserved in parasites, but not in the mammalian host homolog, may represent interaction sites for proteins that are critical for the parasites' viability. The cyclic peptides were synthesized using microwave irradiation to reduce reaction times and increase efficiency. Developing a library of backbone cyclic peptides with different ring sizes facilitates a systematic screen for the most biological active conformation. This method provides a general, fast, and facile way to synthesize cyclic peptides.
Introduction
Protein-protein interactions (PPIs) play a pivotal role in most biological processes, from intracellular signal transduction to cell death1. Hence, targeting PPIs is of fundamental importance to basic research and therapeutic applications. PPIs can be regulated by specific and stable antibodies, but antibodies are expensive and difficult to manufacture and have poor bioavailability. Alternatively, PPIs can be targeted by small molecules. Small molecules are easier to synthesize and inexpensive compared to antibodies; however, they are relatively less flexible and fit better to small cavities than to large protein-protein interfaces2,3. Diverse studies have demonstrated that peptides, which are simpler and cheaper than antibodies and more flexible than small molecules, can bind protein interfaces and regulate PPIs4,5. The global therapeutic peptide market was valued around fifteen billion dollars in 2013 and is growing 10.5% annually6. Furthermore, there are more than 50 marketed peptides, around 270 peptides in different phases of clinical testing, and about 400 peptides in advanced preclinical phases7. Although numerous peptides are being used as drugs, peptides still pose several challenges that limit their widespread application including poor bioavailability and stability, inefficiency in crossing cell membranes, and conformational flexibility8,9. One alternative to surmount these drawbacks is to apply different modifications such as local (D-amino acid and N-alkylation) and global (cyclization) constraints8,10-12. These modifications also occur naturally. For example, cyclosporin A, an immunosuppressant cyclic natural peptide, contains a single D-amino acid and undergoes N-alkylation modifications13,14.
Modification of natural amino acids to induce local constraints, such as D- and N-alkylation, often affects the peptide's biological activity. However, cyclization, in which the sequence of interest can remain the same, is more likely to preserve biological activity. Cyclization is a highly attractive way to restrict peptide conformational space by reducing the equilibrium between different conformations. It usually increases biological activity and selectivity by restricting the peptide to the active conformation that mediates only one function. Cyclization also improves peptide stability by keeping the peptide in a conformation that is less recognized by degrading enzymes. Indeed, cyclic peptides were shown to have improved metabolic stability, bioavailability, and selectivity compared to their linear counterparts15-17.
However, cyclization can be a double-edged sword since in some cases the restriction may prevent the peptides from achieving a bioactive conformation. To overcome this hurdle, a focused library in which all peptides have the same primary sequence and consequently constant pharmacophores can be synthesized. Peptides in the library differ in parameters that influence their structure, such as ring size and position, in order to subsequently screen for the most bioactive conformation9,18.
Peptides can be synthesized both in solution and by a solid-phase peptide synthesis (SPPS) approach, which is now the more prevalent peptide synthesis approach and will be discussed further. SPPS is a process by which chemical transformations are performed on a solid support via a linker to prepare a wide range of synthetic compounds19. SPPS enables assembling peptides by consecutive coupling of amino acids in a stepwise manner from the C-terminus, which is attached to a solid support, to the N-terminus. The N-α-amino acid side-chains must be masked with protecting groups that are stable in the reaction conditions used during peptide elongation to ensure the addition of one amino acid per step. In the final step, the peptide is released from the resin and the side-chain protecting groups are concomitantly removed. While the peptide is being synthesized, all soluble reagents can be removed from the peptide-solid support matrix by filtration and washed away at the end of each coupling step. With such a system, a large excess of reagents at high concentration can drive coupling reactions to completion and all the synthesis steps can be performed in the same vessel without any transfer of material20 .
Although SPPS has some limitations such as the production of incomplete reactions, side reactions, impure reagents, as well as difficulties monitoring the reaction21, the advantages of SPPS have made it the "gold standard" for peptide synthesis. These advantages include the option to incorporate non-natural amino acids, automation, easy purification, minimized physical losses, and the use of excess reagents, resulting in high yields. SPPS has been shown to be extremely useful in the synthesis of difficult sequences21,22, fluorescent modifications23, and peptide libraries24,25. SPPS is also very useful for other poly-chain assemblies such as oligonucleotides26,27, oligosaccharides28,29, and peptide nucleic acids30,31. Interestingly, in some cases, SPPS was shown to be advantageous for synthesizing small molecules that are traditionally made in solution32,33. SPPS is used both in small scale for research and teaching34,35 as well as large scale in industry36-38.
Two synthesis strategies that are mainly used in SPPS methodology for the synthesis of peptides are butyloxycarbonyl (Boc) and 9-fluorenylmethoxycarbonyl (Fmoc). The original strategy introduced for SPPS was Boc, which requires strong acid conditions to remove side-chain protecting groups and cleave the peptide from the resin. Fmoc-based peptide synthesis, however, utilizes moderate base conditions and is a milder alternative to the acid-labile Boc protocol39. The Fmoc strategy utilizes orthogonal t-butyl (tBu) side-chain protection that is removed in the last step of the synthesis while cleaving the peptide from the resin under acid conditions.
The general principle for peptide synthesis on solid support is presented in Figure 1. The initial amino acid, masked by a temporary protecting group on the N-α-terminus, is loaded onto the resin from the C-terminus. A semi-permanent protecting group to mask the side chain is also used if necessary (Figure 1, Step 1). The synthesis of the target peptide is assembled from the C-terminus to the N-terminus by repetitive cycles of deprotection of the N-α-temporary protecting group (Figure 1, Step 2) and coupling of the next protected amino acid (Figure 1, Step 3). After the last amino acid is loaded (Figure 1, Step 4), the peptide is cleaved from the resin support and the semi-permanent protecting groups are removed (Figure 1, Step 5).
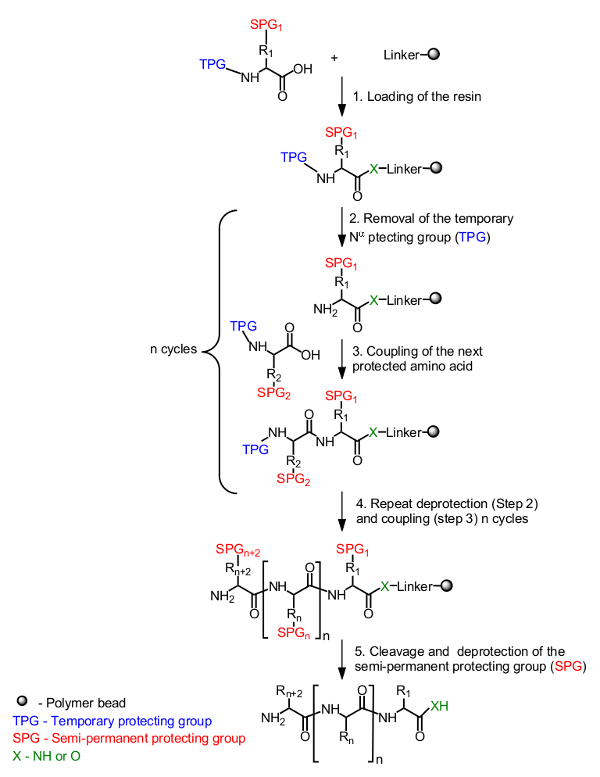
Figure 1. General scheme of solid phase peptide synthesis. The N-α-protected amino acid is anchored using the carboxyl group via a linker to the resin (Step 1). The desired peptide is assembled in a linear fashion from the C-terminus to the N-terminus by repetitive cycles of deprotection of the temporary protecting group (TPG) from the N-α (Step 2) and amino acid coupling (Step 3). After accomplishing the synthesis (Step 4), the semi-permanent protecting groups (SPG) are deprotected during peptide cleavage (Step 5). Please click here to view a larger version of this figure.
After assembly of the complete peptide chain, cyclization can be achieved by several alternatives: (A) head-to-tail cyclization — this is a convenient way but limited since it provides only one option for cyclization (Figure 2A), (B) cyclization using the amino acids from the sequence of interest that contain bioactive functional groups — however, the use of these amino acids may influence the biological activity (Figure 2B), and (C) cyclization by adding amino acids (or other building blocks) without disturbing the bioactive sequence. Introducing these molecules is widespread as it allows production of focused libraries without modifying the sequence of interest (Figure 2C).
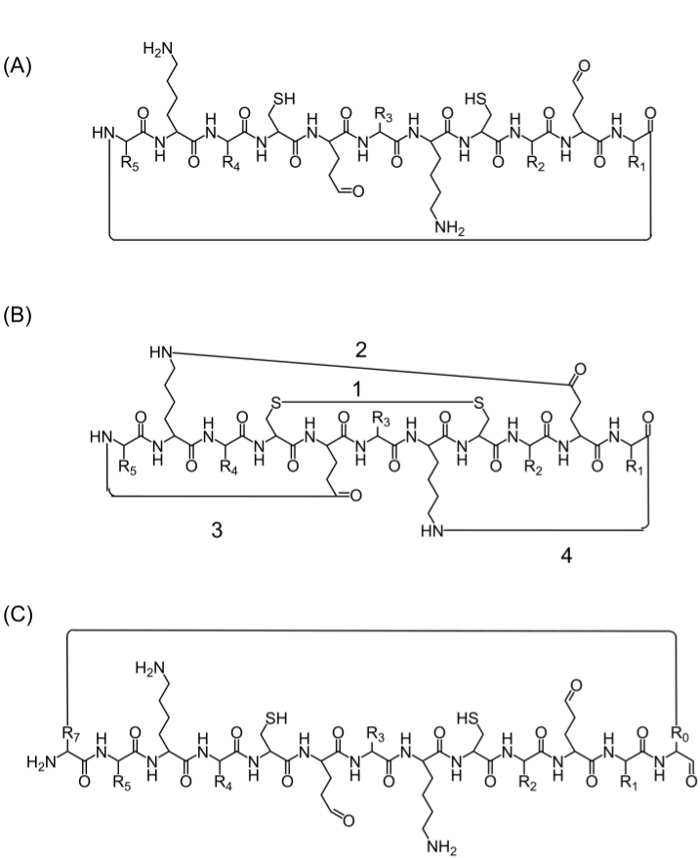
Figure 2. Alternative peptide cyclization strategies. (A) head to tail cyclization, through a peptide bond between the C-terminus and N-terminus; (B) cyclization between functional groups such as a disulfide bond between cysteine residues (1), or an amide bond between the side chains of lysine to aspartic/glutamic acid (2), or side chain to N- or C-terminus (3-4); (C) cyclization by adding extra amino acids or amino acid derivatives or small molecules, for example before (R0) and after (R7) the bioactive sequence. Please click here to view a larger version of this figure.
Microwave-assisted synthesis uses microwave irradiation to heat reactions, thus accelerating organic chemical transformations40,41. Microwave chemistry is based on the ability of the reagent/solvent to absorb the microwave energy and convert it to heat42. Before the technology became widespread, major drawbacks had to be overcome, including the controllability and reproducibility of synthesis protocols and lack of available systems for adequate temperature and pressure controls43,44. The first report of microwave-assisted peptide synthesis was done using a kitchen microwave to synthesize several short peptides (7-10 amino acids) with significant improvement of the coupling efficiency and purity 45. Moreover, microwave energy was shown to decrease chain aggregation, reduce side reactions, limit racemization, and improve coupling rates, which are all critical for difficult and long sequences46-53.
Currently the use of microwave irradiation for the synthesis of peptides or related compounds on a solid support is extensive, including (A) synthesis in water instead of organic solvent54; (B) synthesis of peptides with common post-translational modifications, such as glycopeptides55-58 or phosphopeptides59-61, whose synthesis is typically difficult due to the low coupling efficiency of sterically hindered amino acid derivatives; (C) synthesis of peptides with modification in the backbone, such as azapeptides, which can be formed by the replacement of the C(α) of an amino acid residue with a nitrogen atom62, or peptoids, whose side chain is connected to the amide nitrogen rather than the Cα atom63,64; (D) synthesis of cyclic peptides65-71; and (E) synthesis of combinatorial libraries51,72. In numerous cases, the authors reported higher efficiency and reduced synthesis time using microwave irradiation as compared to the conventional protocol.
Using a rational design73-75, we developed anti-parasitic peptides that were derived from the scaffold Leishmania's receptor for activated C-kinase (LACK). LACK plays an important role in the early phase of Leishmania infection76. Parasites expressing lower levels of LACK fail to parasitize even immune-compromised mice77 as LACK is involved in essential parasite signaling processes and protein synthesis78. Therefore, LACK is a key scaffold protein79 and a valuable drug target. Focusing on sequences in LACK that are conserved in the parasites, but not in the host mammalian homolog RACK, we identified an 8 amino acid peptide (RNGQCQRK) that decreased Leishmania sp. viability in culture.
Here, we describe a protocol for the synthesis of backbone cyclic peptides derived from the LACK protein sequence described above. The peptides were synthesized on a solid support using microwave heating by SPPS methodology with Fmoc/tBu protocol. Peptides were conjugated to a TAT47-57 (YGRKKRRQRRR) carrier peptide through an amide bond as part of the SPPS. TAT-based transport of a variety of cargoes into cells has been used for over 15 years and delivery of the cargo into subcellular organelles has been confirmed80. Four different linkers, succinic and glutaric anhydride as well as adipic and pimelic acid, were used to perform the cyclization to generate carboxylic acid linkers of two to five carbons. Cyclization was done using microwave energy, and the final cleavage and side-chain deprotection steps were done manually without microwave energy. The use of an automated microwave synthesizer improved the product purity, increased the product yield, and reduced the duration of the synthesis. This general protocol can be applied to other studies that utilize peptides to understand important molecular mechanism in vitro and in vivo and further develop potential drugs for human diseases.
Protocol
1. Equipment and Reagents Preparation
- Preparing equipment
- Perform all steps inside a fume hood using proper personal protective equipment.
- Chemically synthesize peptides on solid support using a Microwave Peptide Synthesizer with an additional module of Discover equipped with a fiber-optic temperature probe for controlling the microwave power delivery in a Teflon reaction vessel (30 ml, with a glass frit) or in a disposable polypropylene cartridge (12 ml, with a coarse frit).
- For proper mixing, connect nitrogen supply to the reaction vessel, or alternatively seal both ends of the polypropylene cartridge, and place on a rotary shaker.
- To drain reaction mixtures or washes, connect to the house vacuum via a waste trap.
- Place the fiber-optic probe into the reaction vessel.
- Preparing reagents
- Prepare the resin by weighing Rink Amide AM resin 100-200 mesh (0.204 mg), add 5 ml of 1:1 mixture of N,N-dimethylformamide (DMF)/dichloromethane (DCM) to the reaction vessel/polypropylene cartridge to wash the resin down, shake for 2-4 hr to swell properly, and drain.
- Prepare 0.2 M 9-fluorenylmethoxycarbonyl (Fmoc)-amino acid solutions by dissolving the corresponding Fmoc-amino acid in DMF and vortex the mixture until the amino acids are dissolved (Table 1).
- Prepare 0.45 M activator solution mix by dissolving 18.96 g O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) in 100 ml DMF and vortexing the mixture until the solid is dissolved (Table 1).
- Prepare 2 M activator base solution mix by combining 34.8 ml N,N-diisopropylethylamine (DIEA) with 65.2 ml 1-methyl-2-pyrrolidinone (NMP) (Table 1).
- Prepare 0.1 M deprotection solution mix by dissolving 3.37 g 1-hydroxybenzotriazole hydrate (HOBt) in 250 ml of a 20% v/v solution of piperidine in DMF and vortexing the mixture until the solid is dissolved (Table 1).
2. Fmoc-protected Amino Acid Coupling
- Amino acid coupling
- Add amino acid (2.5 ml)/activator (1 ml)/activator base (0.5 ml) to reaction vessel/polypropylene cartridge and let the reaction proceed for 300 sec (25 W, 75 °C, Table 2). Drain the solution.
- Wash the resin with DMF. Add DMF to the resin for 120 sec (7 ml, 0 W, RT) and drain the solution. Repeat five times.
- Fmoc deprotection
- Add 7 ml of 20% piperidine in DMF with 0.1 M HOBt to reaction vessel/polypropylene cartridge and incubate for 30 sec (45 W, 75 °C, Table 2).
- Drain the reaction mixture.
- Add 7 ml of 20% piperidine in DMF with 0.1 M HOBt to reaction vessel/polypropylene cartridge and incubate for 180 sec (45 W, 75 °C, Table 2).
- Drain the reaction mixture.
- Wash the resin with DMF. Add DMF to the resin for 120 sec (7 ml 0 W, rt) and drain the solution. Repeat five times.
Note: Optionally, pause the procedure here and resume at a later date.
- After amino acid coupling step, wash the resin with DCM and store for at least several days at 4 °C (for a longer period store the resin at – 20 °C).
- Move the resin from the reaction vessel to a polypropylene cartridge.
- Wash the resin with DCM. Add DCM to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat three times.
- Seal the polypropylene cartridge tightly with a top cap and stopcock.
- Before starting a new synthesis, swell the resin for 3-4 hr in DMF (7 ml).
- Monitoring the synthesis
- Use the Kaiser (ninhydrin) test or Chloranil test to quickly determine the progress of the synthesis. Optionally, perform a small-scale cleavage reaction to determine the purity and mass of the synthesized peptide. See section 9.
Note: For further troubleshooting see Table 3.
- Use the Kaiser (ninhydrin) test or Chloranil test to quickly determine the progress of the synthesis. Optionally, perform a small-scale cleavage reaction to determine the purity and mass of the synthesized peptide. See section 9.
- Repeat steps 2.1 and 2.2 as desired to synthesize targeted peptide: Arg(Pbf)-Asn(Trt)-Gly-Gln(Trt)-Cys(Trt)-Gln(Trt)-Arg(Pbf)-Lys(Boc)-Lys(Mtt)-Gly-Gly-Tyr(But)-Gly-Arg(Pbf)-Lys(Boc)-Lys(Boc)-Arg(Pbf)-Arg(Pbf)-Gln(Trt)-Arg(Pbf)-Arg(Pbf)-Arg(Pbf).
3. Anhydride/Acid Coupling
- Anhydride coupling
- Wash the resin with NMP. Add NMP to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat three times.
- Dissolve 10 equivalents of the corresponding anhydride in NMP (5 ml), add 1 equivalent 4-Dimethylaminopyridine (DMAP) and 10 equivalents DIEA to the solution (Table 1).
- Add a 10:1:10 mixture of anhydride/DMAP/DIEA to the resin and incubate for 300 sec (25 W, 75 °C, Table 2). Drain the solution.
- Wash the resin with NMP. Add NMP to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat three times.
- Acid coupling
- Wash the resin with DMF. Add DMF to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat three times.
- Dissolve 10 equivalents of the corresponding dicarboxylic acid in DMF (5 ml). Add 1 equivalent DMAP and 10 equivalents N,N'-Diisopropylcarbodiimide (DIC) to the solution (Table 1).
- Pre-activate the mixture by mixing for 30 min.
- Add the mixture to the resin and incubate for 300 sec (25 W, 75 °C, Table 2) and drain the solution.
- Wash the resin with DMF. Add DMF to the resin for 120 sec (7 ml, 0 W, rt) and drain solution. Repeat step three times.
4. N-methyltrityl (Mtt) Protecting Group Deprotection
Note: The lysine side chain was protected with N-methyltrityl (Mtt)81, a protecting group that can be deprotected selectively under acid labile conditions82,83. Deprotect Mtt protecting group manually on a shaker without microwave energy.
- Transfer the resin to a polypropylene cartridge equipped with cap plug and stopcock.
- Wash the resin with DCM. Add DCM to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat three times.
- Add 15-25 ml of a mixture of 1% Trifluoroacetic acid (TFA), 5% Triisopropylsilane (TIS), and 94% DCM to the polypropylene cartridge per one gram of resin.
Note: TFA is a strong acid and corrosive and is extremely irritating to the skin, eyes, and lung tissue.- Keep concentrated solutions of TFA in the hood at all times.
- Use proper personal protective equipment (eye protection, a lab coat and gloves) and work in a well-ventilated hood. Change gloves promptly if they come in contact with TFA and immediately clean-up any spills. If skin or eyes come in contact with the acid, flush the affected area immediately with water and wash for additional 15 min.
- Place the polypropylene cartridge on a shaker and shake for 5 min at RT.
- Drain the solution from the polypropylene cartridge by applying vacuum.
- Repeat steps 4.3-4.5, three times.
- Wash the resin with DCM. Add DCM to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat five times.
5. Cyclization of the Linear Peptide
- Wash the resin with DCM. Add DCM to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat five times.
- In a 50 ml polypropylene tube, dissolve 5 equivalents Benzotriazole-1-ly-oxy-tris-pyrrolidinophosphonium hexafluorphosphate (PyBOP) in Dibromomethane (DBM, 5 ml) and add 10 equivalents DIEA to the solution (Table 1).
- Add the mixture to the resin and incubate for 300 sec (25 W, 75 °C, Table 2). Drain the solution.
- Wash the resin with DCM. Add DCM to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat three times.
6. Cleavage and Deprotection of Side-chain Groups
- Wash the resin with DCM and diethyl ether.
- Add DCM to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat two times.
- Add diethyl ether to the resin for 120 sec (7 ml, 0 W, rt) and drain the solution. Repeat two times.
- Dry the resin in vacuum desiccator at RT for at least 3 hr over potassium hydroxide (KOH, 1-10 g).
- Weigh the dried resin and transfer it to a polypropylene cartridge.
- Add 10 ml of a pre-cooled trifluoroacetic acid (TFA) cleavage cocktail (e.g., 90% TFA, 2.5% water, 2.5% TIS and 5% phenol) to every one gram of resin.
- Shake for 3 hr at RT.
- Collect the TFA cleavage solution by filtering the resin into a 50 ml polypropylene tube. For the filtration, use the frit that is in the 12 ml polypropylene cartridge.
- Add cold diethyl ether (35 ml) to the tube.
- Centrifuge for 5 min at 1,207 x g at 4 °C.
- Decant the ether layer.
- Repeat Step 6.7-6.9, five times.
7. Drying the Backbone Cyclic Peptide
- Keep the precipitated peptide in the same tube and dry it in a hood for 30 min.
- Dissolve the peptide in a 1:1 mixture of water and acetonitrile (ACN).
- Freeze the final product solution in liquid nitrogen.
- Lyophilize the final product.
8. Characterizing the Backbone Cyclic Peptide
- Dissolve a small sample (1 mg) of the product in water (400 µl).
- Inject the dissolved peptide (10-200 µl) to a reverse phase high-performance liquid chromatography (RP-HPLC) system to test the peptide purity 34.
- Check the mass of the peptide using mass spectrometry matrix-assisted laser desorption ionization (MS-MALDI) 84.
- Mix 1 µl (100 µM) peptide in a 1:1 (v/v) mixture of acetonitrile:water with 1 µl of matrix (5 mg/ml, α-cyano-4-hydroxycinnamic acid) in a 1:1 (v/v) mixture of acetonitrile:water with TFA (0.1%).
- Spot 1 µl on the MS-MALDI plate.
- Dry the sample and place it in the mass spectrometer.
- Weigh the peptide and calculate the percent yield.
- Store at -20 °C.
9. Monitoring the Synthesis
- Kaiser (ninhydrin) test 85
- Prepare the reagent solutions.
- Prepare Solution A by dissolving 16.5 mg of Potassium cyanide (KCN) in 25 ml of distilled water. Dilute 1 ml of the above solution with 49 ml of pyridine.
- Prepare Solution B by dissolving 1 g of ninhydrin in 20 ml of ethanol.
- Prepare Solution C by dissolving 40 g phenol in 20 ml ethanol.
- Use the Kaiser test to check the completion of amino acid coupling or the deprotection of the protecting group.
- Transfer a few beads from the resin to a test tube.
- Add three drops (~ 100 µl) of each solution (A, B and C) and mix.
- Heat the test tube on a heating block at 110 °C for 5 min.
Note: Blue colored beads (positive result) indicate incomplete coupling reaction or deprotection of Fmoc protecting group.
- Prepare the reagent solutions.
- Chloranil test 86
- Prepare the following reagents fresh for each test.
- Prepare a solution of 2% Chloranil in DMF, solution A.
- Prepare a solution of 2% acetaldehyde in DMF, solution B.
- Perform the Chloranil test to check the completion of amino acid coupling or the deprotection of the protecting group.
- Mix 100 µl of solution A with 100 µl of solution B in a 1.5 ml tube.
- Drop the beads in and gently shake for 5 min.
Note: Dark brown colored beads (positive result) indicate deprotection of the Fmoc protecting group or an incomplete coupling reaction.
- Prepare the following reagents fresh for each test.
- Small scale cleavage reaction
- Remove a small amount of resin to a 3 ml polypropylene cartridge equipped with cap plug and stopcock.
- Treat with a 2 ml mixture of 95% TFA, 2.5% water and 2.5% TIS.
- Shake the mixture for 30 min at RT.
- Remove the resin by filtration using the frit of the polypropylene cartridge and evaporate the solvents by a stream of nitrogen.
- Dissolve the residue in water and analyze the product using HPLC and/or MS.
10. Leishmania donovani Promastigote Viability in Culture Assay
- Leishmania donovani (L. donovani) growth and treatment conditions
- Culture L. donovani promastigotes in Dulbecco's Modification of Eagle's Medium (DMEM) with 4.5 g/L glucose, L-glutamine, and sodium pyruvate at 26 °C .
- Treat the L. donovani promastigotes with cyclic peptides (100 µM) for 24 hr at 26 °C.
- Leishmania donovani (L. donovani) viability assay
- Assess parasite viability with 20 µl alamarBlue according to manufacturer's protocol.
- Determine alamarBlue reduction by measuring fluorescence (at 570 nm excitation and 590 nm emission). Higher fluorescence values indicate greater metabolic activity and increased parasite viability.
Representative Results
Here we describe the development of a focused small library of backbone cyclic peptides that specifically target vital PPIs of the Leishmania parasite and act as antiparasitic agents (for review about peptides that target PPIs as antiparasitic agents87). Through the synthesis of novel backbone cyclic peptides, pharmacophores are conserved in a scaffold of extendable size. The strength of the focused library proposed here is the ability to vary peptide scaffold sizes while allowing a restricted degree of conformational freedom through cyclization. The entire synthesis of the backbone cyclic peptides was done using an automated microwave synthesizer on solid support, following the Fmoc/tBu protocol. Cyclization was performed by creating an amide bond between the linker, anhydride/acid, and the side-chain amine of lysine. The final cleavage and side-chain deprotection were carried out manually without microwave energy (for synthesis scheme and final products structure see Figure 3). The product was analyzed by preparative HPLC to yield 25 mg of white powder stored at -20 °C. A sample of the product was checked by MS (Figure 4) and its degree of purity was determined using analytical HPLC (Figure 5). A sample of each cyclic peptide was sent for biological screening. One of the four cyclic peptides (pL1) was active against Leishmania donovani (L. donovani), a parasite causing visceral leishmaniasis, the most severe leishmaniasis in humans. Peptide pL1 reduced parasite viability by 75% as compared with the control treatment (Table 4).
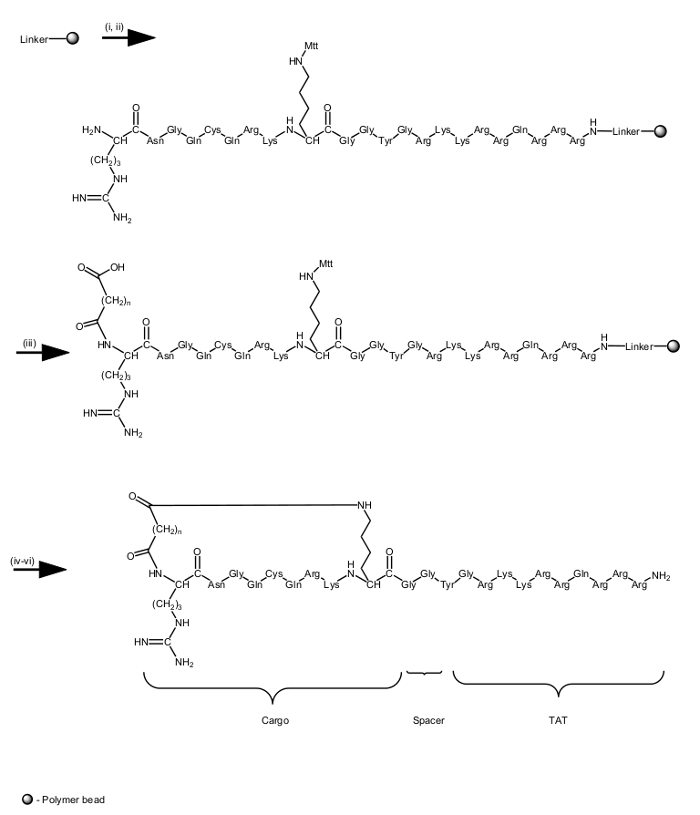
Figure 3. Synthesis scheme and structure of the backbone cyclic peptide synthesized in this study. Reagents and conditions: (i) Amino acid coupling: 300 sec, 25 W, 75 °C, using 1.1:1:2.2 amino acid/activator/activator base. (ii) Fmoc deprotection: 30 sec and 180 sec both at 45 W, 75 °C, using 20% piperidine in DMF + 0.1 M HOBt. (iii) Anhydride coupling: 300 sec, 25 W, 75 °C, using 10:10:1 anhydride/DIEA/DMAP in NMP. (iv) Mtt deprotection: 3 * (300 sec, 0 W, rt) using 1:5:94 TFA/TIS/DCM. (v) Cyclization: 300 sec, 25 W, 75 °C, using 5:10 PyBOP/DIEA in DBM. (vi) Cleavage and deprotection: 3 h, 0 W, rt, using 90:2.5: 2.5:5 TFA/TIS/H2O/Phenol. Peptides were conjugated to a TAT47-57 (Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg) carrier peptide through an amide bond as part of the solid phase synthesis. Please click here to view a larger version of this figure.
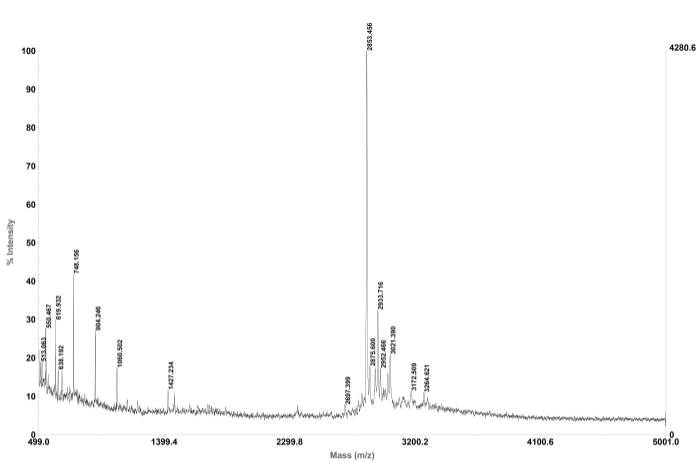
Figure 4. MALDI-TOF mass spectroscopy trace of representative backbone cyclic peptide. The observed mass, 2853.456 is in close agreement to the calculated mass, 2854.271. Please click here to view a larger version of this figure.
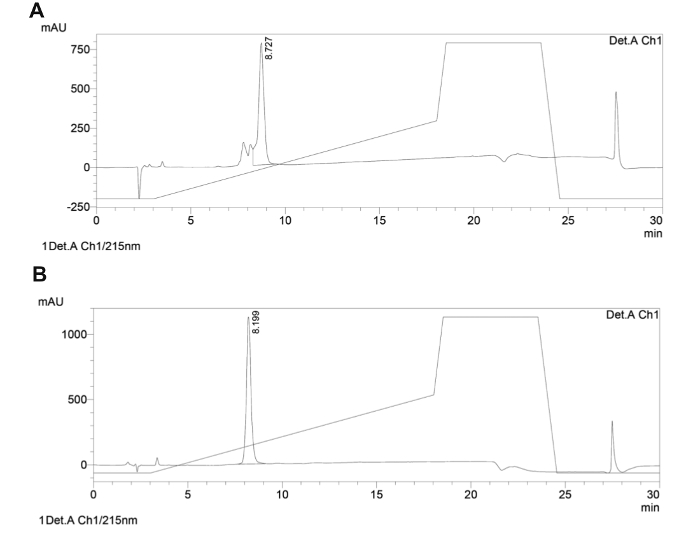
Figure 5. Analytical reverse phase HPLC trace of representative backbone cyclic peptide. The analytical HPLC traces of the crude (A) and purified (B) backbone cyclic peptide are shown. The solvent systems used were A (H2O with 0.1% TFA) and B (CH3CN with 0.1% TFA). A linear gradient of 5-50% B at 1 ml/min in 15 min at 40 °C with a C18, 5 µm, 150 mm column was applied and the detection was at 215 nm. Please click here to view a larger version of this figure.
| Solution | Reagent | MW (g/mol) | d (g/ml) | Volume (ml) | Concentration (M) | Total Amount |
| – Amino acid solution – | Alanine amino acid | 311.34 | 0.2 | 6.23 g | ||
| 0.2 M of amino acid in DMF | DMF | 100 | 100 ml | |||
| An example for alanine amino acid, but the same calculation should be done for each amino acid, with the appropriate MW. To prepare a 100 ml amino acid solution dissolve 6.23 g of alanine amino acid in 100 ml DMF. Store at 4 °C. | ||||||
| – Deprotection solution – | HOBt | 135.1 | 0.1 | 3.37 g | ||
| 20% v/v solution of piperidine in DMF with 0.1M HOBt | Piperidine | 50 | 50 ml | |||
| DMF | 200 | 200 ml | ||||
| Deprotection is used for removal of the Fmoc Nα – protecting group. To prepare a 250 ml deprotection solution dissolve 3.37 g HOBt in 200 ml DMF and add 50 ml piperidine. Store at 4 °C. | ||||||
| – Activator solution – | HBTU | 379.24 | 0.45 | 18.96 g | ||
| 0.45 M HBTU in DMF | DMF | 100 | 100 ml | |||
| Activator is used with the activator base to activate the amino acid before the coupling reaction. To prepare a 100 ml activator solution dissolve 18.96 g HBTU in 100 ml DMF. Store at 4 °C. | ||||||
| – Activator base solution – | DIEA | 129.24 | 0.742 | 2 | 34.80 ml | |
| 2 M DIEA in NMP | NMP | 65.20 ml | ||||
| Activator base is used with the activator to activate the amino acid before the coupling reaction. To prepare a 100 ml activator base solution mix 34.8 ml DIEA and 65.2 ml NMP. Store at 4 °C. | ||||||
| Solution | Reagent | MW (g/mol) | d (g/ml) | Volume (ml) | Eq | Total Amount |
| Anhydride solution — 10:1:10 anhydride/DMAP/DIEA in NMP | Glutaric/Succinic anhydride | 114.1/ 100.07 | 10 | 0.11/0.10 g | ||
| DMAP | 122.2 | 1 | 0.01 g | |||
| DIEA | 129.24 | 0.742 | 10 | 0.09 ml | ||
| NMP | 5 | 5 ml | ||||
| Dissolve 0.11/0.10 g of glutaric/succinic anhydride in 5 ml NMP, add 0.01 g of DMAP and 0.09 ml of DIEA to the solution. Prepare a fresh solution. | ||||||
| Acid solution — 10:1:10 acid/DMAP/DIC in DMF | Adipic/Pimelic acid | 146.14/160.17 | 10 | 0.15/0.16 g | ||
| DMAP | 122.2 | 1 | 0.01 g | |||
| DIC | 126.2 | 0.806 | 10 | 0.16 ml | ||
| DMF | 5 | 5 ml | ||||
| Dissolve 0.15/0.16 g of Adipic/Pimelic acid in 5 ml DMF, add 0.01 g of DMAP and 0.16 ml of DIC to the solution. Prepare a fresh solution. | ||||||
| Cyclization solution — 5:10 PyBOP/DIEA in DBM | PyBOP | 520.3 | 5 | 0.26 g | ||
| DIEA | 129.24 | 0.742 | 10 | 0.09 ml | ||
| DBM | 5 ml | 5 ml | ||||
| Dissolve 0.26 g PyBOP in 5 ml DBM and add 0.09 ml of DIEA to the solution. Prepare a fresh solution. | ||||||
Table 1. Reagents and solutions for the backbone cyclic peptide synthesis. List of the solutions and reagents for the synthesis is provided.
| Microwave cycle | Power (Watts) | Temp (°C ) | Time (sec) | ||
| 1 | Coupling amino acids | 25 | 75 | 300 | |
| 2 | Deprotection of Fmoc protecting group | (a) Initial deprotection | 45 | 75 | 30 |
| (b) Complete deprotection | 45 | 75 | 180 | ||
Table 2. Microwave cycles for coupling and deprotection. Microwave cycles for amino acid coupling and Fmoc-deprotection. (1) Coupling of amino acids. (2) Deprotection of the Fmoc masking group is done in two steps: (a) initial and (b) complete deprotection.
| Problem | Possible reason | Solution |
| Kaiser or Chloranil tests are positive after amino acid coupling | The amino acid coupling is incomplete | Repeat the coupling step |
| Peptides are not efficiently separated from the supernatant | Excess amount of TFA | Evaporate the sample using a stream of nitrogen |
| Presence of deletion sequences in the product | Fmoc removal is incomplete | Monitor the deprotection by Kaiser or Chloranil tests and/or small scale cleavage, in case the Fmoc removal is incomplete repeat the step |
| Amino acid coupling is incomplete | Monitor the coupling by Kaiser or Chloranil tests and/or small scale cleavage, in case the amino acid coupling is incomplete repeat the step and/or use longer reaction time |
Table 3. Troubleshooting advice List of solutions for the most common synthetic challenges is provided.
| Peptide | Sequence | n | MS. Cal. | MS Obs. | HPLC | Yield | Parasite viability | ||
| pL1 | RNGQCQRK-GG-YGRKKRRQRRR | 2 | 2854.321 | 2853.456 | 98% | 86% | 25% | ||
| pL2 | RNGQCQRK-GG-YGRKKRRQRRR | 3 | 2868.348 | 2868.808 | 98% | 87% | 100% | ||
| pL3 | RNGQCQRK-GG-YGRKKRRQRRR | 4 | 2882.375 | 2881.823 | 96% | 89% | 97% | ||
| pL4 | RNGQCQRK-GG-YGRKKRRQRRR | 5 | 2896.402 | 2895.603 | 97% | 85% | 98% | ||
Table 4. Characterization and bioactivity of the peptides in this study. n refers to the number of methylenes in the alkyl spacer (see Figure 3 for structure). MS was done using MALDI technique and purity was determined by analytical HPLC. Peptides were added to Leishmania donovani promastigotes and the viability of parasites was assessed and expressed as percent survival relative to control cultures incubated in the absence of peptide. Only pL1 had high Leishmanicidal activity. The observer was blinded to the experimental conditions. Data are representative of three independent experiments.
Discussion
The synthesis of a focused library of backbone cyclic peptides derived from the LACK protein of the Leishmania parasite using a fully automated microwave synthesizer is described. A focused library of cyclic peptides was developed with conserved pharmacophores and various linkers. Addition of various linkers such as glutaric anhydride, succinic anhydride, adipic acid, pimelic acid, lysine, ornithine, and other building blocks can be used to increase the variety of the conformational space of the cyclic peptides. The synthesis of a focused cyclic peptides library allows researchers to screen for the optimal conformational space. Since the conformation of cyclic peptides varies depending on parameters such as ring size and position, diverse analogs with different conformations can be generated, which may be useful in biological structure-activity relationship studies88.
A main challenge in SPPS is diagnosing the synthetic progress and problem-solving since no intermediates are isolated. Therefore, several colorimetric tests can be used to monitor the reaction, such as those that identify free amines by Kaiser and Chloranil tests. If the Kaiser or Chloranil tests are not indicative (e.g., proline and hydroxy-proline do not react with ninhydrin in the same way as the other amino acids because their alpha amino group is part of a five membered ring), a small scale cleavage reaction and mass spectrometry analysis may be applied to monitor synthesis progress.
Cleavage time and the cleavage cocktail can be modified based on the chemical properties and number of the protecting groups used. It is recommended that an initial trial cleavage using a small amount of the resin (1-10 mg) be performed to verify the proper conditions. King et al. have tested different cleavage cocktails for various peptides and their detailed guidelines can be used to optimize reaction conditions89. For backbone cyclic peptides, incubation for at least 3 hr is recommended as a default for full cleavage. However, peptides containing a high number of protecting groups or difficult protecting groups (e.g., t-butyl ester or pentamethyl-2,3-dihydrobenzofuran-5-sulfonyl) should be incubated for a longer time to ensure complete deprotection. Herein, we have not systematically studied the optimal cleavage time or cocktail. Nevertheless, we found that a short cleavage time (less than 2 hr) resulted in incomplete cleavage of some protecting groups.
The standard microwave peptide synthesis protocol is a generally applicable method for the synthesis of a variety of peptides. In most cases, the use of an automatic microwave synthesizer reduces the synthesis duration and increases the yield and purity of the products. Furthermore, it decreases side reactions such as racemization and aspartimide formation. Although we have not done a side-by-side comparison of microwave and conventional synthesis in this study, based on our and other labs' experience, it was shown that the use of microwave-assisted synthesis is superior to the conventional protocol61,70. Almost all activators and resins can be effectively used in microwave SPPS and the general method can also be applied to the synthesis of a variety of modified peptides, such as, glycopeptides, phosphopeptides, azapeptides, peptoids, and cyclic peptides90.
Cyclization is a convenient way to enhance the potency and stability of linear precursors. Cyclic peptides can obtain a desirable constrained conformation that may contribute to increased binding affinity and selectivity . Furthermore, linear peptides can be modified to contain multiple cyclic loops, enabling them to possibly target multiple endogenous protein binding interfaces 91. However, it is important to note that cyclization does not necessarily lead to improvements in all or sometimes any of these properties. Certain cyclic peptides can result in conformations that are not recognized by targeted receptors (for example92,93).Therefore, a focused library of cyclic peptides is necessary to screen for bioactivity. In conclusion, synthetic cyclic peptides exhibit desirable pharmacological characteristics, are small enough to cross the cell membrane, and are large enough to have high selectivity. High potency, specificity, and safe profile contribute to cyclic peptides' promise as drug candidates.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Lauren Van Wassenhove, Sunhee Hwang, and Daria Mochly-Rosen for helpful discussions. The work was supported by the National Institutes of Health Grant NIH RC4 TW008781-01 C-IDEA (SPARK) to N.Q. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materials
| REAGENTS | |||
| Solid support, Rink Amide AM resin ML | CBL | BR-1330 | loading: 0.49 mmol/g |
| Fmoc-Ala-OH | Advanced Chemtech | FA2100 | |
| Fmoc-Arg(Pbf)-OH | Advanced Chemtech | FR2136 | |
| Fmoc-Asn(Trt)-OH | Advanced Chemtech | FN2152 | |
| Fmoc-Asp(OBut)-OH | Advanced Chemtech | FD2192 | |
| Fmoc-Cys(Trt)-OH | Advanced Chemtech | FC2214 | |
| Fmoc-Gln(Trt)-OH | Advanced Chemtech | FQ2251 | |
| Fmoc-Glu(OtBu)-OH | Advanced Chemtech | FE2237 | |
| Fmoc-Gly-OH | Advanced Chemtech | FG2275 | |
| Fmoc-His(Trt)-OH | Advanced Chemtech | FH2316 | |
| Fmoc-Ile-OH | Advanced Chemtech | FI2326 | |
| Fmoc-Leu-OH | Advanced Chemtech | FL2350 | |
| Fmoc-Lys(Boc)-OH | Advanced Chemtech | FK2390 | |
| Fmoc-Met-OH | Advanced Chemtech | FM2400 | |
| Fmoc-Phe-OH | Advanced Chemtech | FF2425 | |
| Fmoc-Pro-OH | Advanced Chemtech | FP2450 | |
| Fmoc-Ser-(tBu)-OH | Advanced Chemtech | FS2476 | |
| Fmoc-Thr(tBu)-OH | Advanced Chemtech | FT2518 | |
| Fmoc-Trp(Boc)-OH | Advanced Chemtech | FW2527 | |
| Fmoc-Tyr(But)-OH | Advanced Chemtech | FY2563 | |
| Fmoc-Val-OH | Advanced Chemtech | FV2575 | |
| 1-Methyl-2-pyrrolidinone (NMP) | Sigma | 328634 | Caution Toxic/Highly flammable/Irritant. |
| N,N-Dimethylformamide (DMF) | Alfa Aesar | 43465 | Caution Toxic |
| Use high quality DMF to eliminate side reactions such as Fmoc removal as a result of the dimethylamine traces from DMF decomposition. | |||
| Dichloromethane (DCM) | Sigma | D65100 | Caution Harmful |
| Dibromomethane (DBM) | Sigma | D41868 | Caution Harmful |
| Trifluoroacetic acid (TFA) | Sigma | T62200 | Caution Corrosive/Toxic |
| Trifluoroacetic acid, HPLC grade (TFA) | Sigma | 91707 | Caution Corrosive/Toxic |
| Diethylether | Sigma | 31690 | Caution Highly flammable/Harmful |
| Triisopropylsilane (TIS) | Sigma | 233781 | Caution Irritant/Flammable |
| Water, HPLC grade | Sigma | 270733 | |
| Acetonitroile, HPLC grade (ACN) | Fisher Scientific | A998-4 | Caution Flammable/Irritant/Harmful |
| N,N-Diisopropylethylamine (DIEA) | Sigma | 3440 | Caution Corrosive/Highly flammable |
| Piperidine | Sigma | W290807 | Caution Toxic/Highly flammable |
| Pyridine | Sigma | 270970 | Caution Highly flammable/Harmful |
| Ethanol (EtOH) | Sigma | 459844 | Caution Highly flammable/Irritant |
| 1-Hydroxybenzotriazole hydrate (HOBt) | Sigma | 157260 | Caution Highly flammable/Irritant/Harmful |
| O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) | Sigma | 12804 | Caution Irritant/Harmful |
| Benzotriazole-1-ly-oxy-tris-pyrrolidinophosphonium hexafluorphosphate (PyBOP) | Advanced Chemtech | RC8602 | Caution Irritant |
| Ninhydrin | Sigma | 454044 | Caution Harmful |
| Phenol | Sigma | P3653 | Caution Corrosive/Toxic |
| Potassium cyanide (KCN) | Sigma | 11813 | Caution Very Toxic |
| Potassium hydroxide (KOH) | Sigma | 221473 | Caution Toxic |
| N,N’- | Sigma | 38370 | Caution Flammable/ Toxic |
| Diisopropylcarbodiimide (DIC) | |||
| 4-Dimethylaminopyridine (DMAP) | Sigma | 522805 | Caution Toxic/Irritant |
| Glutaric anhydride | Sigma | G3806 | Caution Flammable/Irritant/Harmful |
| Succinic anhydride | Sigma | 239690 | Caution Irritant/Harmful |
| Adipic acid | Sigma | A26357 | Caution Toxic/Irritant |
| Pimelic acid | Sigma | P45001 | Caution Toxic/Irritant |
| Chloranil | Sigma | 23290 | Caution Toxic/Irritant |
| Acetaldehyde | Sigma | 402788 | Caution Flammable/ Toxic |
| EQUIPMENT | |||
| Name | Company | Catalog Number | Comments |
| Centrifuge | Beckman Coulter | Allegra 6R centrifuge | |
| Lyophilizer | Labconco | freezone 4.5 | |
| Vacuum pump | Franklin Electric | model 1101101416 with 3/4 HP | Alcatel pump with Franklin Motor |
| Polypropylene cartridge 12 ml | Applied Separation | 2419 | |
| Cap plug for 12 ml polypropylene cartridge | Applied Separation | 8157 | |
| Polypropylene cartridge 3 ml | Applied Separation | 2413 | |
| Cap plug for 3 ml polypropylene cartridge | Applied Separation | 8054 | |
| Stop cocks PTFE | Applied Separation | 2406 | |
| Tubes flat, 50 ml | VWR | 21008-240 | |
| Extraction manifold, 20 pos, 16 x 100 mm tubes | Waters | WAT200609 | |
| Shaker, BD adams™ nutator mixer | Fisher scientific | 22363152 | |
| Nalgene HDPE narrow mouth IP2 bottles, 125 ml | Fisher scientific | 03-312-8 | |
| Erlenmeyer flask | Fisher Scientific | FB-501, 500 ml | |
| Heating block | Thermolyne | 1760 dri bath | |
| Disposable borosilicate glass tubes with plain end | Fisher Scientific | 14-961-25 | |
| Micropipettes and tips Finnpipette | Thermo | 20–200 and 100–1,000 μl | |
| HPLC vials – micro vl pp 400 µl PK100 | VWR | 69400-124 | |
| HPLC vial- Blue Snap-It Cap | VWR | 66030-600 | |
| Analytical HPLC column | Peeke Scientific | U1-5C18Q-JJ | ultro 120 5 µm C18Q, 4.6 mm ID 150 mm |
| Prep HPLC column, XBridge | Waters | OBD C18 5 µm column | 19 mm × 150 mm |
| Mass spectrometer | Applied Biosystems | Voyager DE-RP |
References
- Wells, J. A., McClendon, C. L. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 450 (7172), 1001-1009 (2007).
- Arkin, M. R., Wells, J. A. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. (4), 301-317 (2004).
- Mandell, D. J., Kortemme, T. Computer-aided design of functional protein interactions. Nat. Chem. Biol. 5 (11), 797-807 (2009).
- Friedler, A., et al. Backbone cyclic peptide, which mimics the nuclear localization signal of human immunodeficiency virus type 1 matrix protein, inhibits nuclear import and virus production in nondividing cells. Biochimie. 37 (16), 5616-5622 (1998).
- Brandman, R., Disatnik, M. H., Churchill, E., Mochly-Rosen, D. Peptides derived from the C2 domain of protein kinase C epsilon (epsilon PKC) modulate epsilon PKC activity and identify potential protein-protein interaction surfaces. J. Biol. Chem. 282 (6), 4113-4123 (2007).
- Vlieghe, P., Lisowski, V., Martinez, J., Khrestchatisky, M. Synthetic therapeutic peptides: science and market. Drug discov today. 15 (1-2), 40-56 (2010).
- Marx, V. Watching Peptide Drugs Grow Up. Chemical & Engineering News. 83, 17-24 (2005).
- Denicourt, C., Dowdy, S. F. Medicine. Targeting apoptotic pathways in cancer cells. Science. 305 (5689), 1411-1413 (2004).
- Qvit, N., et al. Synthesis of a novel macrocyclic library: discovery of an IGF-1R inhibitor. J Comb Chem. 10 (2), 256-266 (2008).
- Patch, J. A., Barron, A. E. Mimicry of bioactive peptides via non-natural, sequence-specific peptidomimetic oligomers. Curr. Opin. Chem. Biol. 6 (6), 872-877 (2002).
- Kessler, H. Peptide Conformations .19. Conformation and Biological-Activity of Cyclic-Peptides. Angew. Chem. Int. Ed. Engl. 21 (7), 512-523 (1982).
- Gazal, S., Gelerman, G., Gilon, C. Novel Gly building units for backbone cyclization: synthesis and incorporation into model peptides. Peptides. 24 (12), 1847-1852 (2003).
- Fesik, S. W., et al. NMR studies of [U-13C]cyclosporin A bound to cyclophilin: bound conformation and portions of cyclosporin involved in binding. Biochimie. 30 (26), 6574-6583 (1991).
- Kornfeld, O. S., et al. Mitochondrial Reactive Oxygen Species at the Heart of the Matter: New Therapeutic Approaches for Cardiovascular Diseases. Circ. Res. 116 (11), 1783-1799 (2015).
- Boguslavsky, V., Hruby, V. J., O’Brien, D. F., Misicka, A., Lipkowski, A. W. Effect of peptide conformation on membrane permeability. J. Pept. Res. 61 (6), 287-297 (2003).
- Eguchi, M., et al. Solid-phase synthesis and structural analysis of bicyclic beta-turn mimetics incorporating functionality at the i to i+3 positions. J. Am. Chem. Soc. 121 (51), 12204-12205 (1999).
- Altstein, M., et al. Backbone cyclic peptide antagonists, derived from the insect pheromone biosynthesis activating neuropeptide, inhibit sex pheromone biosynthesis in moths. J. Biol. Chem. 274 (25), 17573-17579 (1999).
- Cheng, M. F., Fang, J. M. Liquid-phase combinatorial synthesis of 1,4-benzodiazepine-2,5-diones as the candidates of endothelin receptor antagonism. J. Comb. Chem. 6 (1), 99-104 (2004).
- Merrifield, R. B. Solid Phase Peptide Synthesis I. the Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 85, 2149-2154 (1963).
- Pfeiffer, C. T., Schafmeister, C. E. Solid phase synthesis of a functionalized bis-peptide using ‘safety catch’ methodology. J Vis Exp. (63), e4112 (2012).
- Coin, I., Beyermann, M., Bienert, M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2 (12), 3247-3256 (2007).
- Qvit, N., et al. Design and synthesis of backbone cyclic phosphorylated peptides: the IκB model. Biopolymers. 91 (2), 157-168 (2009).
- Sainlos, M., Imperiali, B. Tools for investigating peptide-protein interactions: peptide incorporation of environment-sensitive fluorophores through SPPS-based ‘building block’ approach. Nat. Protoc. 2 (12), 3210-3218 (2007).
- Hilpert, K., Winkler, D. F., Hancock, R. E. Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat. Protoc. 2 (6), 1333-1349 (2007).
- Qi, X., Qvit, N., Su, Y. C., Mochly-Rosen, D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J. Cell Sci. 126 (Pt 3), 789-802 (2013).
- Beaucage, S. L. Solid-phase synthesis of siRNA oligonucleotides. Curr. Opin. Drug Discovery Dev. 11 (2), 203-216 (2008).
- Dhanawat, M., Shrivastava, S. K. Solid-Phase Synthesis of Oligosaccharide Drugs: A Review. Mini Rev Med Chem. 9 (2), 169-185 (2009).
- Seeberger, P. H., Werz, D. B. Synthesis and medical applications of oligosaccharides. Nature. 446 (7139), 1046-1051 (2007).
- Plante, O. J., Palmacci, E. R., Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science. 291 (5508), 1523-1527 (2001).
- Komiyama, M., Aiba, Y., Ishizuka, T., Sumaoka, J. Solid-phase synthesis of pseudo-complementary peptide nucleic acids. Nat. Protoc. 3 (4), 646-654 (2008).
- Christensen, L., et al. Solid-Phase synthesis of peptide nucleic acids. J. Pept. Sci. 1 (3), 175-183 (1995).
- Qvit, N., et al. Development of bifunctional photoactivatable benzophenone probes and their application to glycoside substrates. Biopolymers. 90 (4), 526-536 (2008).
- O’Neill, J. C., Blackwell, H. E. Solid-phase and microwave-assisted syntheses of 2,5-diketopiperazines: small molecules with great potential. Comb Chem High Throughput Screen. 10 (10), 857-876 (2007).
- Qvit, N., Barda, Y., Shalev, D., Gilon, C. A Laboratory Preparation of Aspartame Analogs Using Simultaneous Multiple Parallel Synthesis Methodology. J. Chem. Educ. 84 (12), 1988-1991 (2007).
- Truran, G. A., Aiken, K. S., Fleming, T. R., Webb, P. J., Markgraf, J. H. Solid phase organic synthesis and combinatorial chemistry: A laboratory preparation of oligopeptides. J. Chem. Educ. 79 (1), 85-86 (2002).
- Verlander, M. Industrial applications of solid-phase peptide synthesis – A status report. Int. J. Pept. Res. Ther. 13 (1-2), 75-82 (2007).
- Bray, B. L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nature reviews. Drug discovery. 2 (7), 587-593 (2003).
- Qvit, N. Development and therapeutic applications of oligonucleotides and peptides. chimica Oggi / CHEMISTRY today. 29 (2), 4-7 (2011).
- Carpino, L. A., Han, G. Y. 9-Fluorenylmethoxycarbonyl Amino-Protecting Group. J. Org. Chem. 37 (22), 3404-3409 (1972).
- Gedye, R., et al. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 27 (3), 279-282 (1986).
- Giguere, R. J., Bray, T. L., Duncan, S. M., Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 27 (41), 4945-4948 (1986).
- Kappe, C. O., Dallinger, D. The impact of microwave synthesis on drug discovery. Nature reviews. Drug discovery. 5 (1), 51-63 (2006).
- Kappe, C. O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. Engl. 43 (46), 6250-6284 (2004).
- de la Hoz, A., Diaz-Ortiz, A., Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 34 (2), 164-178 (2005).
- Yu, H. M., Chen, S. T., Wang, K. T. Enhanced coupling efficiency in solid-phase peptide synthesis by microwave irradiation. J. Org. Chem. 57 (18), 4781-4784 (1992).
- Mingos, D. M. P., Baghurst, D. R. Tilden Lecture. Applications of microwave dielectric heating effects to synthetic problems in chemistry. Chem. Soc. Rev. 20 (1), 1-47 (1991).
- Gabriel, C., Gabriel, S., Grant, E. H., Halstead, B. S. J., Mingos, D. M. P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 27 (3), 213-224 (1998).
- Sabatino, G., Papini, A. M. Advances in automatic, manual and microwave-assisted solid-phase peptide synthesis. Curr. Opin. Drug Discovery Dev. 11 (6), 762-770 (2008).
- Banerjee, J., Hanson, A. J., Muhonen, W. W., Shabb, J. B., Mallik, S. Microwave-assisted synthesis of triple-helical, collagen-mimetic lipopeptides. Nat. Protoc. 5 (1), 39-50 (2010).
- Bacsa, B., Kappe, C. O. Rapid solid-phase synthesis of a calmodulin-binding peptide using controlled microwave irradiation. Nat. Protoc. 2 (9), 2222-2227 (2007).
- Murray, J. K., Gellman, S. H. Parallel synthesis of peptide libraries using microwave irradiation. Nat. Protoc. 2 (3), 624-631 (2007).
- Palasek, S. A., Cox, Z. J., Collins, J. M. Limiting racemization and aspartimide formation in microwave-enhanced Fmoc solid phase peptide synthesis. J Pept Sci. 13 (3), 143-148 (2007).
- Murray, J. K., Aral, J., Miranda, L. P. Solid-Phase Peptide Synthesis Using Microwave Irradiation. Methods Mol. Biol. 716, 73-88 (2011).
- Galanis, A. S., Albericio, F., Grotli, M. Solid-Phase Peptide Synthesis in Water Using Microwave-Assisted Heating. Organic Letters. 11 (20), 4488-4491 (2009).
- Rizzolo, F., Sabatino, G., Chelli, M., Rovero, P., Papini, A. M. A convenient microwave-enhanced solid-phase synthesis of difficult peptide sequences: Case study of Gramicidin A and CSF114(Glc). Int. J. Pept. Res. Ther. 13 (1-2), 203-208 (2007).
- Matsushita, T., Hinou, H., Kurogochi, M., Shimizu, H., Nishimura, S. Rapid microwave-assisted solid-phase glycopeptide synthesis. Org Lett. 7 (5), 877-880 (2005).
- Nagaike, F., et al. Efficient microwave-assisted tandem N- to S-acyl transfer and thioester exchange for the preparation of a glycosylated peptide thioester. Org Lett. 8 (20), 4465-4468 (2006).
- Naruchi, K., et al. Construction and structural characterization of versatile lactosaminoglycan-related compound library for the synthesis of complex glycopeptides and glycosphingolipids. J. Org. Chem. 71 (26), 9609-9621 (2006).
- Brandt, M., Gammeltoft, S., Jensen, K. J. Microwave heating for solid-phase peptide synthesis: General evaluation and application to 15-mer phosphopeptides. Int. J. Pept. Res. Ther. 12 (4), 349-357 (2006).
- Harris, P. W. R., Williams, G. M., Shepherd, P., Brimble, M. A. The Synthesis of Phosphopeptides Using Microwave-assisted Solid Phase Peptide Synthesis. Int. J. Pept. Res. Ther. 14 (4), 387-392 (2008).
- Qvit, N. Microwave-assisted Synthesis of Cyclic Phosphopeptide on Solid Support. Chem. Biol. Drug Des. 85 (3), 300-305 (2014).
- Kato, D., Verhelst, S. H., Sexton, K. B., Bogyo, M. A general solid phase method for the preparation of diverse azapeptide probes directed against cysteine proteases. Org Lett. 7 (25), 5649-5652 (2005).
- Olivos, H. J., Alluri, P. G., Reddy, M. M., Salony, D., Kodadek, T. Microwave-assisted solid-phase synthesis of peptoids. Org Lett. 4 (23), 4057-4059 (2002).
- Gorske, B. C., Jewell, S. A., Guerard, E. J., Blackwell, H. E. Expedient synthesis and design strategies for new peptoid construction. Org Lett. 7 (8), 1521-1524 (2005).
- Grieco, P., et al. Design and microwave-assisted synthesis of novel macrocyclic peptides active at melanocortin receptors: discovery of potent and selective hMC5R receptor antagonists. J. Med. Chem. 51 (9), 2701-2707 (2008).
- Boutard, N., Jamieson, A. G., Ong, H., Lubell, W. D. Structure-Activity Analysis of the Growth Hormone Secretagogue GHRP-6 by alpha- and beta-Amino gamma-Lactam Positional Scanning. Chem. Biol. Drug Des. 75 (1), 40-50 (2010).
- Jamieson, A. G., et al. Positional scanning for peptide secondary structure by systematic solid-phase synthesis of amino lactam peptides. J. Am. Chem. Soc. 131 (22), 7917-7927 (2009).
- Hossain, M. A., Bathgate, R. A. D., Tregear, G., Wade, J. D. De Novo Design and Synthesis of Cyclic and Linear Peptides to Mimic the Binding Cassette of Human Relaxin. Annals of the New York Academy of Sciences. 1160, 16-19 (2009).
- Fowler, S. A., Stacy, D. M., Blackwell, H. E. Design and synthesis of macrocyclic peptomers as mimics of a quorum sensing signal from Staphylococcus aureus. Org Lett. 10 (12), 2329-2332 (2008).
- Cemazar, M., Craik, D. J. Microwave-assisted Boc-solid phase peptide synthesis of cyclic cysteine-rich peptides. J Pept Sci. 14 (6), 683-689 (2008).
- Miles, S. M., Leatherbarrow, R. J., Marsden, S. P., Coates, W. J. Synthesis and bio-assay of RCM-derived Bowman-Birk inhibitor analogues. Org Biomol Chem. 2 (3), 281-283 (2004).
- Murray, J. K., et al. Efficient synthesis of a beta-peptide combinatorial library with microwave irradiation. J. Am. Chem. Soc. 127 (38), 13271-13280 (2005).
- Churchill, E. N., Qvit, N., Mochly-Rosen, D. Rationally designed peptide regulators of protein kinase. C. Trends Endocrinol. Metab. 20 (1), 25-33 (2009).
- Mochly-Rosen, D., Qvit, N. Peptide inhibitors of protein-protein interactions. chimica Oggi / CHEMISTRY today. 28 (1), 14-16 (2010).
- Qvit, N., Mochly-Rosen, D. Highly specific modulators of protein kinase C localization: applications to heart failure. Drug Discov. Today Dis. Mech. 7 (2), e87-e93 (2010).
- Mougneau, E., et al. Expression cloning of a protective Leishmania antigen. Science. 268 (5210), 563-566 (1995).
- Kelly, B. L., Stetson, D. B., Locksley, R. M. Leishmania major LACK antigen is required for efficient vertebrate parasitization. J. Exp. Med. 198 (11), 1689-1698 (2003).
- Choudhury, K., et al. Trypanosomatid RACK1 orthologs show functional differences associated with translation despite similar roles in Leishmania pathogenesis. PLoS One. 6 (6), e20710 (2011).
- Gonzalez-Aseguinolaza, G., Taladriz, S., Marquet, A., Larraga, V. Molecular cloning, cell localization and binding affinity to DNA replication proteins of the p36/LACK protective antigen from Leishmania infantum. Eur. J. Biochem. 259 (3), 909-916 (1999).
- Gump, J. M., Dowdy, S. F. TAT transduction: the molecular mechanism and therapeutic prospects. Trends Mol. Med. 13 (10), 443-448 (2007).
- Aletras, A., Barlos, K., Gatos, D., Koutsogianni, S., Mamos, P. Preparation of the very acid-sensitive Fmoc-Lys(Mtt)-OH. Application in the synthesis of side-chain to side-chain cyclic peptides and oligolysine cores suitable for the solid-phase assembly of MAPs and TASPs. Int. J. Pept. Protein Res. 45 (5), 488-496 (1995).
- Li, D., Elbert, D. L. The kinetics of the removal of the N-methyltrityl (Mtt) group during the synthesis of branched peptides. J. Pept. Res. 60 (5), 300-303 (2002).
- Bourel, L., Carion, O., Gras-Masse, H., Melnyk, O. The deprotection of Lys(Mtt) revisited. J Pept Sci. 6 (6), 264-270 (2000).
- Tran, H., Gael, S. L., Connolly, M. D., Zuckermann, R. N. Solid-phase submonomer synthesis of peptoid polymers and their self-assembly into highly-ordered nanosheets. J Vis Exp. (57), e3373 (2011).
- Kaiser, E., Colescot, R. L., Bossinge, C. D., Cook, P. I. Color Test for Detection of Free Terminal Amino Groups in Solid-Phase Synthesis of Peptides. Anal. Biochem. 34 (2), 595-598 (1970).
- Christensen, T. Qualitative Test for Monitoring Coupling Completeness in Solid-Phase Peptide-Synthesis Using Chloranil. Acta Chem. Scand. Ser.B-Org. Chem. Biochem. 33 (10), 763-766 (1979).
- Qvit, N., Crapster, J. A. Peptides that Target Protein-Protein Interactions as an Anti-Parasite Strategy. chimica Oggi / CHEMISTRY today. 32 (6), 62-66 (2014).
- Byk, G., et al. Synthesis and biological activity of NK-1 selective, N-backbone cyclic analogs of the C-terminal hexapeptide of substance P. J. Med. Chem. 39 (16), 3174-3178 (1996).
- King, D. S., Fields, C. G., Fields, G. B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int. J. Pept. Protein Res. 36 (3), 255-266 (1990).
- Pedersen, S. L., Tofteng, A. P., Malik, L., Jensen, K. J. Microwave heating in solid-phase peptide synthesis. Chemical Society Reviews. 41 (5), 1826-1844 (2012).
- Colangelo, A. M., et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J. Neurosci. 28 (11), 2698-2709 (2008).
- Mesfin, F. B., Andersen, T. T., Jacobson, H. I., Zhu, S., Bennett, J. A. Development of a synthetic cyclized peptide derived from alpha-fetoprotein that prevents the growth of human breast cancer. J. Pept. Res. 58 (3), 246-256 (2001).
- Mizejewski, G. J., Muehlemann, M., Dauphinee, M. Update of alpha fetoprotein growth-inhibitory peptides as biotherapeutic agents for tumor growth and metastasis. Chemotherapy. 52 (2), 83-90 (2006).

