一个主干环肽库作为潜在的抗寄生虫治疗微波辐射的发展
Summary
A simple and general method for the synthesis of cyclic peptides using microwave irradiation is outlined. This procedure enables the synthesis of backbone cyclic peptides with a collection of different conformations while retaining the side chains and the pharmacophoric moieties., and therefore, allows to screen for the bioactive conformation.
Abstract
蛋白质 – 蛋白质相互作用(质子泵抑制剂)是密切参与几乎所有的生物过程,并与许多人类疾病。因此,有一个重大努力的目标在基础研究和在制药工业中质子泵抑制剂。蛋白质 – 蛋白质接口通常是大而平的,而且往往缺乏口袋,小分子靶向此类网站的发现复杂化。使用抗体靶向的替代方法有由于不良的口服生物利用度,低格,透气性好,生产效率低下的限制。
使用的肽靶向PPI接口具有几个优点。肽具有更高的构象柔性,提高的选择性,并且通常便宜。然而,肽具有其自身的局限性,包括稳定性差,效率低下穿越细胞膜。为了克服这种局限性,肽的环化可以被执行。环化已被证明提高肽选择性,代谢稳定性,和生物利用率。然而,预测环肽的生物活性的构象是不平凡的。为了克服这一难题,人们有吸引力的方法是筛选一个焦点库到屏幕,其中所有主链的环肽具有相同的一级序列,但是不同之处在于影响其构象的参数,如环的大小和位置。
我们描述了一个详细的协议用于合成靶向特定寄生虫质子泵抑制剂骨干环肽的文库。采用合理的设计方法,我们开发了从支架L蛋白eishmania受体激活C-激酶(LACK)的肽。我们假设,即是保守的寄生虫,但不是在哺乳动物宿主同源的拉克序列,可以表示相互作用位点的蛋白质,是对于寄生虫'能力至关重要。合成的环肽是使用微波照射以减少反应时间和增加效率。开发骨干环肽具有不同的戒指尺寸库便于系统的屏幕为大多数生物活性构象。此方法提供合成环肽的一般,快速和容易的方式。
Introduction
蛋白质-蛋白质相互作用(质子泵抑制剂)起到最生物过程的关键作用,从细胞内的信号转导至细胞死亡1。因此,针对生产者价格指数是具有根本的重要性,以基础研究和治疗应用。质子泵抑制剂可以通过特定的和稳定的抗体来调节,但抗体是昂贵的并且难以制造,并且具有生物利用度差。另外,质子泵抑制剂可通过小分子靶向。小分子更容易合成和廉价相比抗体;然而,它们是相对不灵活和更适合于细孔比大的蛋白质-蛋白质界面2,3。多样的研究表明,肽,它们比抗体简单和更便宜,比小分子更加灵活,可以结合蛋白接口和调节质子泵抑制剂4,5。全球治疗肽市场总值约1,500十亿美元在2013年增长10.5%禾LLY 6。此外,有50多个市售的肽,周围270肽在临床试验的不同阶段,和大约400肽在先进的临床前阶段7。尽管众多的肽被用作药物,肽仍然构成限制其广泛的应用,包括生物利用度差和稳定性,低效率在交叉细胞膜,和构象柔性8,9-几个挑战。一个替代方案克服这些缺点是应用不同的修改,例如本地(D-氨基酸和N-烷基化)和全局(环化)约束8,10-12。这些修改也自然出现。例如,环孢菌素A,免疫抑制剂的环肽天然,包含一个单一的D-氨基酸和经历N-烷基化修饰13,14。
修饰的天然氨基酸以诱导本地约束,如D-和N-烷基化,通常会影响所述肽9氏生物活性。然而,环化,其中感兴趣的序列可以保持不变,是更可能保留生物活性。环化是一个非常有吸引力的方式通过减少不同的构象之间的平衡,以限制肽构象的空间。它通常通过限制肽与活性构象,其介导只有一个函数增加生物活性和选择性。环化也通过保持肽在较少受到降解酶识别的构象提高肽的稳定性。事实上,环肽均表现相比相应的线性15-17具有改善的代谢稳定性,生物利用度,和选择性。
然而,环化可以是一把双刃剑,因为在某些情况下,限制可能阻止肽从达到的生物活性构象。为了克服这一障碍,集中库中的所有肽具有相同的主sequencË因此不断的药效可以合成。在库中的肽的不同在于,影响其结构参数,如环的大小和位置,以便随后筛选最生物活性的构象9,18。
肽可以既在溶液中并通过固相肽合成(SPPS)的方式,也就是现在的更普遍的肽合成方法,并将进一步讨论的合成。 SPPS是通过该化学转化通过接头连接在固体载体上进行,以制备多种合成化合物19的方法。 SPPS使组装肽由氨基酸连续耦合在从C末端,其连接到一个固体支持物以逐步的方式,以N-末端。的N-α-氨基酸侧链必须与保护是稳定在期间延伸肽使用的反应条件组,以确保在加入每ST一个氨基酸被掩蔽EP。在最后的步骤中,将肽从树脂释放,侧链保护基团伴随除去。而该肽被合成,所有的可溶性试剂可以通过过滤从肽 – 固体载体基质被移除,并在每个偶联步骤的末尾冲走。有了这样的系统,在高浓度的大过量的试剂可以驱动偶合反应至完成,所有的合成步骤可以在同一个容器中无材料 20的任何传输来执行。
虽然SPPS具有如生产不完全反应,副反应,不纯的试剂,以及困难监测反应21的一些局限,SPPS的优点已经使它的“金标准”的肽合成。这些优点包括选项掺入非天然氨基酸,自动化,容易纯化,最小化物理损失,以及使用过量的试剂,导致高收益。 SPPS已被证明是在困难的序列21,22,荧光修饰23,和肽文库24,25的合成极为有用。 SPPS也是其他聚链组件,例如寡核苷酸26,27,寡糖28,29,和肽核酸30,31非常有用的。有趣的是,在某些情况下,SPPS被证明是有利的,用于合成被在溶液32,33传统上制成的小分子。 SPPS可以用在小规模的研究和教学34,35以及大规模产业36-38。
这主要应用于SPPS方法为肽合成两种合成策略是氧羰基(BOC)和9-芴基(Fmoc)。引入SPPS原始策略是Boc时,这需要强酸条件从第r除去侧链保护基团和裂解肽ESIN。将Fmoc-基于肽合成,不过,使用中等强度的碱的条件,是对酸不稳定的Boc协议39较温和的替代方案。将Fmoc策略利用了设置在合成的最后步骤中除去而裂解从在酸性条件下的树脂肽正交叔丁基(TBU)侧链保护。
对于在固体载体上的肽合成的一般原则是在图1的初始氨基酸,通过在N-α-末端的临时保护基团掩盖,被装载到从C末端的树脂。甲半永久性保护基掩蔽侧链还用于在必要时(图1,步骤1)。靶肽的合成是从C末端组装到N末端 的N-α-临时保护基的脱保护的重复循环(图1,步骤2)和耦合下一保护的氨基酸(图1 </str翁>,步骤3)。后最后一个氨基酸被加载(图1,步骤4),将肽从树脂载体切下并半永久性保护基团被除去(图1,步骤5)。
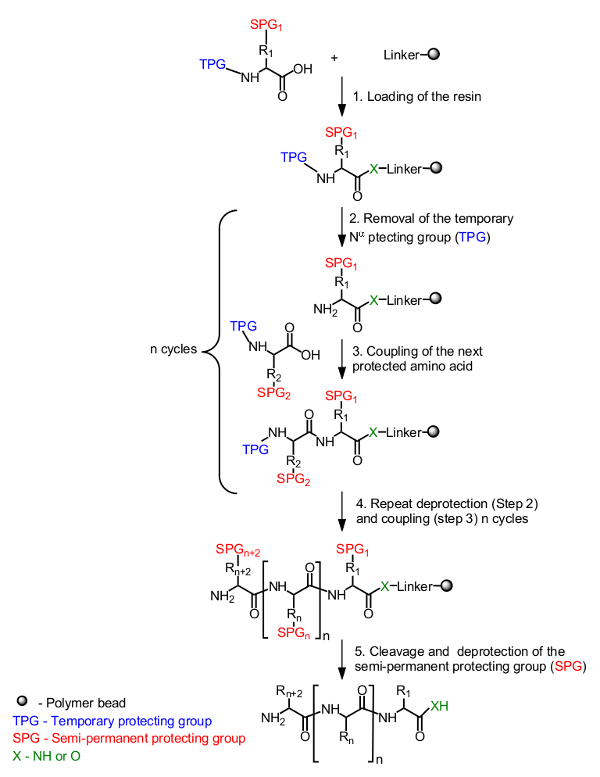
的固相肽合成图 1.常规 方案的 N-α-保护的氨基酸通过接头与树脂(步骤1)使用羧基锚定。所需的肽组装在从C末端到N末端通过从N-α(步骤2)和氨基酸耦合(步骤3)的临时保护基团(TPG)的脱保护的重复循环以线性方式。完成的合成(步骤4)后,将半永久性保护基(SPG)的过程中肽裂解(步骤5)去保护。获得=“_空白”>点击此处查看该图的放大版本。
完整的肽链的组装后,环化可以通过多种替代来实现:(A)的头-尾环化-这是一种方便的方式,但不限于,因为它仅提供一个用于环化(图2A)的选项,(B)的环化使用来自感兴趣序列中的氨基酸,包含生物活性官能团的-然而,使用这些氨基酸可通过,而不会干扰增加的氨基酸(或其他构件块)的影响的生物活性(图2B),和(C)环化生物活性序列。引入这些分子是普遍,因为它允许生产集中的库,而无需修改的兴趣(图2C)的序列。
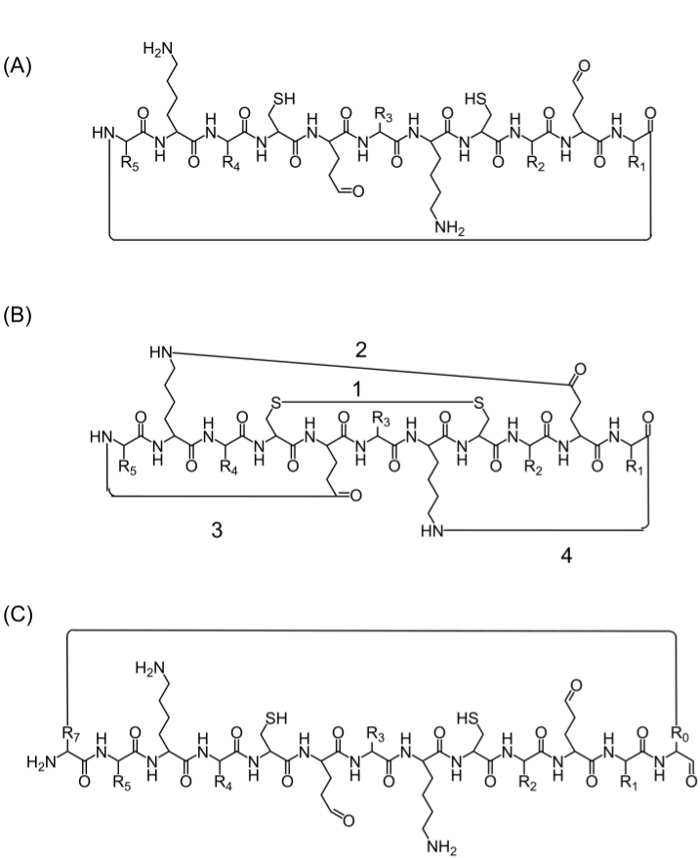
Figure 2. 可替代的肽环化法 (A)的头至尾环化,通过C末端和N末端 之间的肽键。官能团之间(B)的环化,如半胱氨酸残基(1),或赖氨酸的侧链之间形成酰胺键之间的二硫键来天冬氨酸/谷氨酸(2),或侧链为N-或C-末端(3 -4); (C)环化通过增加额外的氨基酸或氨基酸衍生物或小分子,例如前(R0)和(R7)的生物活性序列之后, 请点击此处查看该图的放大版本。
微波辅助合成采用微波辐射加热反应,从而加速有机化工转化40,41。微波化学是基于试剂/能力溶剂吸收所微波能量并将其转换成热42。前的技 术变得普遍,主要缺点必须克服,包括可控性和合成协议再现性和缺乏可用的系统为适当的温度和压力控制的43,44。做使用厨房微波合成几个短肽(7-10个氨基酸)与显著提高耦合效率和纯度45的微波辅助肽合成的第一份报告。此外,微波能量被证明减少链聚集,降低副反应,限制了外消旋化,并提高连接速度,这是所有艰难和漫长的序列46-53的关键。
目前在固体载体上使用微波照射于肽或相关化合物的合成中是广泛的,包括(A)中合成的有机溶剂54的水代替; (B)的合成肽的同常见的翻译后修饰,如糖 肽55-58或59-61磷酸,其合成通常是困难的,因为位阻氨基酸衍生物的低耦合效率; (C)的合成与修饰肽主链,如azapeptides,其可以由替换的氨基酸残基的C(α)与氮原子62,或拟肽,其侧链连接到被形成的酰胺氮而非Cα原子63,64; (d)合成环肽65-71的;和(E)的合成组合库51,72中。在许多情况下,作者报告更高的效率和降低的合成时间使用微波辐射相比于常规协议。
用合理的设计73-75,我们开发均来自支架大号 eishmania的受体FO抗寄生虫肽- [R激活C-激酶(LACK)。拉克起着利什曼原虫属感染76的早期阶段发挥重要作用。寄生虫表达缺乏较低水平不缺乏参与必不可少的寄生虫信号传导过程和蛋白质的合成78寄生甚至免疫缺陷小鼠77。因此,缺乏的是一个关键的支架蛋白79和宝贵的药物靶点。重点放在中保守的寄生虫,但不是在宿主哺乳动物同系物的RACK在拉克序列,我们确定了一个8个氨基酸的肽(RNGQCQRK),在培养降低利什曼原虫属的生存能力。
在这里,我们描述了一种协议,用于从上述的LACK蛋白序列衍生骨架的环肽的合成。合成使用微波加热通过SPPS方法用Fmoc / TBU协议的坚实支持的肽。肽是通过酰胺键缀合到一个TAT 47-57(YGRKKRRQRRR)载体肽在SPPS的一部分。的各种货物到细胞中的TAT-基于运输已经使用了超过15年,并输送货物到亚细胞器已经证实80。四种不同的接头,琥珀酸和戊二酸酐以及己二酸和庚二酸,被用来进行环化,以产生二至五个碳原子的羧酸的接头。已完成的环化利用微波能量,和没有微波能量被手动完成的最终裂解和侧链去保护步骤。使用一个自动化微波合成器中的改进的产品纯度,增加了产品的产量,并降低了合成的持续时间。这是一般的协议可以被应用到利用肽理解重要的分子机制在体外和体内 ,并进一步发展潜力的药物用于人类疾病的其他研究。
Protocol
Representative Results
Discussion
从使用一个完全自动化的微波合成的利什曼原虫的蛋白质缺乏衍生骨干环肽的焦点库的合成描述。环肽的焦点库与保守的药效和各种接头的发展。加入各种接头如戊二酸酐,琥珀酸酐,己二酸,庚二酸,赖氨酸,鸟氨酸,和其他构件块的可用于增加品种的环肽的构象空间。有重点的环肽库的合成,使研究人员筛选最佳的构象空间。因为环肽的构象的变化取决于参数例如环的尺寸和位置,可?…
Divulgations
The authors have nothing to disclose.
Acknowledgements
我们感谢劳伦凡沃森霍夫,Sunhee黄某和达里亚Mochly – 罗森有益的讨论。这项工作是支持的健康资助NIH RC4 TW008781-01 C-IDEA(SPARK)全国学院与NQ的资助者在研究设计,数据收集和分析,发布决定,或准备的手稿没有作用。
Materials
| REAGENTS | |||
| Solid support, Rink Amide AM resin ML | CBL | BR-1330 | loading: 0.49 mmol/g |
| Fmoc-Ala-OH | Advanced Chemtech | FA2100 | |
| Fmoc-Arg(Pbf)-OH | Advanced Chemtech | FR2136 | |
| Fmoc-Asn(Trt)-OH | Advanced Chemtech | FN2152 | |
| Fmoc-Asp(OBut)-OH | Advanced Chemtech | FD2192 | |
| Fmoc-Cys(Trt)-OH | Advanced Chemtech | FC2214 | |
| Fmoc-Gln(Trt)-OH | Advanced Chemtech | FQ2251 | |
| Fmoc-Glu(OtBu)-OH | Advanced Chemtech | FE2237 | |
| Fmoc-Gly-OH | Advanced Chemtech | FG2275 | |
| Fmoc-His(Trt)-OH | Advanced Chemtech | FH2316 | |
| Fmoc-Ile-OH | Advanced Chemtech | FI2326 | |
| Fmoc-Leu-OH | Advanced Chemtech | FL2350 | |
| Fmoc-Lys(Boc)-OH | Advanced Chemtech | FK2390 | |
| Fmoc-Met-OH | Advanced Chemtech | FM2400 | |
| Fmoc-Phe-OH | Advanced Chemtech | FF2425 | |
| Fmoc-Pro-OH | Advanced Chemtech | FP2450 | |
| Fmoc-Ser-(tBu)-OH | Advanced Chemtech | FS2476 | |
| Fmoc-Thr(tBu)-OH | Advanced Chemtech | FT2518 | |
| Fmoc-Trp(Boc)-OH | Advanced Chemtech | FW2527 | |
| Fmoc-Tyr(But)-OH | Advanced Chemtech | FY2563 | |
| Fmoc-Val-OH | Advanced Chemtech | FV2575 | |
| 1-Methyl-2-pyrrolidinone (NMP) | Sigma | 328634 | Caution Toxic/Highly flammable/Irritant. |
| N,N-Dimethylformamide (DMF) | Alfa Aesar | 43465 | Caution Toxic |
| Use high quality DMF to eliminate side reactions such as Fmoc removal as a result of the dimethylamine traces from DMF decomposition. | |||
| Dichloromethane (DCM) | Sigma | D65100 | Caution Harmful |
| Dibromomethane (DBM) | Sigma | D41868 | Caution Harmful |
| Trifluoroacetic acid (TFA) | Sigma | T62200 | Caution Corrosive/Toxic |
| Trifluoroacetic acid, HPLC grade (TFA) | Sigma | 91707 | Caution Corrosive/Toxic |
| Diethylether | Sigma | 31690 | Caution Highly flammable/Harmful |
| Triisopropylsilane (TIS) | Sigma | 233781 | Caution Irritant/Flammable |
| Water, HPLC grade | Sigma | 270733 | |
| Acetonitroile, HPLC grade (ACN) | Fisher Scientific | A998-4 | Caution Flammable/Irritant/Harmful |
| N,N-Diisopropylethylamine (DIEA) | Sigma | 3440 | Caution Corrosive/Highly flammable |
| Piperidine | Sigma | W290807 | Caution Toxic/Highly flammable |
| Pyridine | Sigma | 270970 | Caution Highly flammable/Harmful |
| Ethanol (EtOH) | Sigma | 459844 | Caution Highly flammable/Irritant |
| 1-Hydroxybenzotriazole hydrate (HOBt) | Sigma | 157260 | Caution Highly flammable/Irritant/Harmful |
| O-(Benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) | Sigma | 12804 | Caution Irritant/Harmful |
| Benzotriazole-1-ly-oxy-tris-pyrrolidinophosphonium hexafluorphosphate (PyBOP) | Advanced Chemtech | RC8602 | Caution Irritant |
| Ninhydrin | Sigma | 454044 | Caution Harmful |
| Phenol | Sigma | P3653 | Caution Corrosive/Toxic |
| Potassium cyanide (KCN) | Sigma | 11813 | Caution Very Toxic |
| Potassium hydroxide (KOH) | Sigma | 221473 | Caution Toxic |
| N,N’- | Sigma | 38370 | Caution Flammable/ Toxic |
| Diisopropylcarbodiimide (DIC) | |||
| 4-Dimethylaminopyridine (DMAP) | Sigma | 522805 | Caution Toxic/Irritant |
| Glutaric anhydride | Sigma | G3806 | Caution Flammable/Irritant/Harmful |
| Succinic anhydride | Sigma | 239690 | Caution Irritant/Harmful |
| Adipic acid | Sigma | A26357 | Caution Toxic/Irritant |
| Pimelic acid | Sigma | P45001 | Caution Toxic/Irritant |
| Chloranil | Sigma | 23290 | Caution Toxic/Irritant |
| Acetaldehyde | Sigma | 402788 | Caution Flammable/ Toxic |
| EQUIPMENT | |||
| Name | Company | Catalog Number | Comments |
| Centrifuge | Beckman Coulter | Allegra 6R centrifuge | |
| Lyophilizer | Labconco | freezone 4.5 | |
| Vacuum pump | Franklin Electric | model 1101101416 with 3/4 HP | Alcatel pump with Franklin Motor |
| Polypropylene cartridge 12 ml | Applied Separation | 2419 | |
| Cap plug for 12 ml polypropylene cartridge | Applied Separation | 8157 | |
| Polypropylene cartridge 3 ml | Applied Separation | 2413 | |
| Cap plug for 3 ml polypropylene cartridge | Applied Separation | 8054 | |
| Stop cocks PTFE | Applied Separation | 2406 | |
| Tubes flat, 50 ml | VWR | 21008-240 | |
| Extraction manifold, 20 pos, 16 x 100 mm tubes | Waters | WAT200609 | |
| Shaker, BD adams™ nutator mixer | Fisher scientific | 22363152 | |
| Nalgene HDPE narrow mouth IP2 bottles, 125 ml | Fisher scientific | 03-312-8 | |
| Erlenmeyer flask | Fisher Scientific | FB-501, 500 ml | |
| Heating block | Thermolyne | 1760 dri bath | |
| Disposable borosilicate glass tubes with plain end | Fisher Scientific | 14-961-25 | |
| Micropipettes and tips Finnpipette | Thermo | 20–200 and 100–1,000 μl | |
| HPLC vials – micro vl pp 400 µl PK100 | VWR | 69400-124 | |
| HPLC vial- Blue Snap-It Cap | VWR | 66030-600 | |
| Analytical HPLC column | Peeke Scientific | U1-5C18Q-JJ | ultro 120 5 µm C18Q, 4.6 mm ID 150 mm |
| Prep HPLC column, XBridge | Waters | OBD C18 5 µm column | 19 mm × 150 mm |
| Mass spectrometer | Applied Biosystems | Voyager DE-RP |
References
- Wells, J. A., McClendon, C. L. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 450 (7172), 1001-1009 (2007).
- Arkin, M. R., Wells, J. A. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov. (4), 301-317 (2004).
- Mandell, D. J., Kortemme, T. Computer-aided design of functional protein interactions. Nat. Chem. Biol. 5 (11), 797-807 (2009).
- Friedler, A., et al. Backbone cyclic peptide, which mimics the nuclear localization signal of human immunodeficiency virus type 1 matrix protein, inhibits nuclear import and virus production in nondividing cells. Biochimie. 37 (16), 5616-5622 (1998).
- Brandman, R., Disatnik, M. H., Churchill, E., Mochly-Rosen, D. Peptides derived from the C2 domain of protein kinase C epsilon (epsilon PKC) modulate epsilon PKC activity and identify potential protein-protein interaction surfaces. J. Biol. Chem. 282 (6), 4113-4123 (2007).
- Vlieghe, P., Lisowski, V., Martinez, J., Khrestchatisky, M. Synthetic therapeutic peptides: science and market. Drug discov today. 15 (1-2), 40-56 (2010).
- Marx, V. Watching Peptide Drugs Grow Up. Chemical & Engineering News. 83, 17-24 (2005).
- Denicourt, C., Dowdy, S. F. Medicine. Targeting apoptotic pathways in cancer cells. Science. 305 (5689), 1411-1413 (2004).
- Qvit, N., et al. Synthesis of a novel macrocyclic library: discovery of an IGF-1R inhibitor. J Comb Chem. 10 (2), 256-266 (2008).
- Patch, J. A., Barron, A. E. Mimicry of bioactive peptides via non-natural, sequence-specific peptidomimetic oligomers. Curr. Opin. Chem. Biol. 6 (6), 872-877 (2002).
- Kessler, H. Peptide Conformations .19. Conformation and Biological-Activity of Cyclic-Peptides. Angew. Chem. Int. Ed. Engl. 21 (7), 512-523 (1982).
- Gazal, S., Gelerman, G., Gilon, C. Novel Gly building units for backbone cyclization: synthesis and incorporation into model peptides. Peptides. 24 (12), 1847-1852 (2003).
- Fesik, S. W., et al. NMR studies of [U-13C]cyclosporin A bound to cyclophilin: bound conformation and portions of cyclosporin involved in binding. Biochimie. 30 (26), 6574-6583 (1991).
- Kornfeld, O. S., et al. Mitochondrial Reactive Oxygen Species at the Heart of the Matter: New Therapeutic Approaches for Cardiovascular Diseases. Circ. Res. 116 (11), 1783-1799 (2015).
- Boguslavsky, V., Hruby, V. J., O’Brien, D. F., Misicka, A., Lipkowski, A. W. Effect of peptide conformation on membrane permeability. J. Pept. Res. 61 (6), 287-297 (2003).
- Eguchi, M., et al. Solid-phase synthesis and structural analysis of bicyclic beta-turn mimetics incorporating functionality at the i to i+3 positions. J. Am. Chem. Soc. 121 (51), 12204-12205 (1999).
- Altstein, M., et al. Backbone cyclic peptide antagonists, derived from the insect pheromone biosynthesis activating neuropeptide, inhibit sex pheromone biosynthesis in moths. J. Biol. Chem. 274 (25), 17573-17579 (1999).
- Cheng, M. F., Fang, J. M. Liquid-phase combinatorial synthesis of 1,4-benzodiazepine-2,5-diones as the candidates of endothelin receptor antagonism. J. Comb. Chem. 6 (1), 99-104 (2004).
- Merrifield, R. B. Solid Phase Peptide Synthesis I. the Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 85, 2149-2154 (1963).
- Pfeiffer, C. T., Schafmeister, C. E. Solid phase synthesis of a functionalized bis-peptide using ‘safety catch’ methodology. J Vis Exp. (63), e4112 (2012).
- Coin, I., Beyermann, M., Bienert, M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2 (12), 3247-3256 (2007).
- Qvit, N., et al. Design and synthesis of backbone cyclic phosphorylated peptides: the IκB model. Biopolymers. 91 (2), 157-168 (2009).
- Sainlos, M., Imperiali, B. Tools for investigating peptide-protein interactions: peptide incorporation of environment-sensitive fluorophores through SPPS-based ‘building block’ approach. Nat. Protoc. 2 (12), 3210-3218 (2007).
- Hilpert, K., Winkler, D. F., Hancock, R. E. Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat. Protoc. 2 (6), 1333-1349 (2007).
- Qi, X., Qvit, N., Su, Y. C., Mochly-Rosen, D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J. Cell Sci. 126 (Pt 3), 789-802 (2013).
- Beaucage, S. L. Solid-phase synthesis of siRNA oligonucleotides. Curr. Opin. Drug Discovery Dev. 11 (2), 203-216 (2008).
- Dhanawat, M., Shrivastava, S. K. Solid-Phase Synthesis of Oligosaccharide Drugs: A Review. Mini Rev Med Chem. 9 (2), 169-185 (2009).
- Seeberger, P. H., Werz, D. B. Synthesis and medical applications of oligosaccharides. Nature. 446 (7139), 1046-1051 (2007).
- Plante, O. J., Palmacci, E. R., Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science. 291 (5508), 1523-1527 (2001).
- Komiyama, M., Aiba, Y., Ishizuka, T., Sumaoka, J. Solid-phase synthesis of pseudo-complementary peptide nucleic acids. Nat. Protoc. 3 (4), 646-654 (2008).
- Christensen, L., et al. Solid-Phase synthesis of peptide nucleic acids. J. Pept. Sci. 1 (3), 175-183 (1995).
- Qvit, N., et al. Development of bifunctional photoactivatable benzophenone probes and their application to glycoside substrates. Biopolymers. 90 (4), 526-536 (2008).
- O’Neill, J. C., Blackwell, H. E. Solid-phase and microwave-assisted syntheses of 2,5-diketopiperazines: small molecules with great potential. Comb Chem High Throughput Screen. 10 (10), 857-876 (2007).
- Qvit, N., Barda, Y., Shalev, D., Gilon, C. A Laboratory Preparation of Aspartame Analogs Using Simultaneous Multiple Parallel Synthesis Methodology. J. Chem. Educ. 84 (12), 1988-1991 (2007).
- Truran, G. A., Aiken, K. S., Fleming, T. R., Webb, P. J., Markgraf, J. H. Solid phase organic synthesis and combinatorial chemistry: A laboratory preparation of oligopeptides. J. Chem. Educ. 79 (1), 85-86 (2002).
- Verlander, M. Industrial applications of solid-phase peptide synthesis – A status report. Int. J. Pept. Res. Ther. 13 (1-2), 75-82 (2007).
- Bray, B. L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nature reviews. Drug discovery. 2 (7), 587-593 (2003).
- Qvit, N. Development and therapeutic applications of oligonucleotides and peptides. chimica Oggi / CHEMISTRY today. 29 (2), 4-7 (2011).
- Carpino, L. A., Han, G. Y. 9-Fluorenylmethoxycarbonyl Amino-Protecting Group. J. Org. Chem. 37 (22), 3404-3409 (1972).
- Gedye, R., et al. The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett. 27 (3), 279-282 (1986).
- Giguere, R. J., Bray, T. L., Duncan, S. M., Majetich, G. Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 27 (41), 4945-4948 (1986).
- Kappe, C. O., Dallinger, D. The impact of microwave synthesis on drug discovery. Nature reviews. Drug discovery. 5 (1), 51-63 (2006).
- Kappe, C. O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. Engl. 43 (46), 6250-6284 (2004).
- de la Hoz, A., Diaz-Ortiz, A., Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 34 (2), 164-178 (2005).
- Yu, H. M., Chen, S. T., Wang, K. T. Enhanced coupling efficiency in solid-phase peptide synthesis by microwave irradiation. J. Org. Chem. 57 (18), 4781-4784 (1992).
- Mingos, D. M. P., Baghurst, D. R. Tilden Lecture. Applications of microwave dielectric heating effects to synthetic problems in chemistry. Chem. Soc. Rev. 20 (1), 1-47 (1991).
- Gabriel, C., Gabriel, S., Grant, E. H., Halstead, B. S. J., Mingos, D. M. P. Dielectric parameters relevant to microwave dielectric heating. Chem. Soc. Rev. 27 (3), 213-224 (1998).
- Sabatino, G., Papini, A. M. Advances in automatic, manual and microwave-assisted solid-phase peptide synthesis. Curr. Opin. Drug Discovery Dev. 11 (6), 762-770 (2008).
- Banerjee, J., Hanson, A. J., Muhonen, W. W., Shabb, J. B., Mallik, S. Microwave-assisted synthesis of triple-helical, collagen-mimetic lipopeptides. Nat. Protoc. 5 (1), 39-50 (2010).
- Bacsa, B., Kappe, C. O. Rapid solid-phase synthesis of a calmodulin-binding peptide using controlled microwave irradiation. Nat. Protoc. 2 (9), 2222-2227 (2007).
- Murray, J. K., Gellman, S. H. Parallel synthesis of peptide libraries using microwave irradiation. Nat. Protoc. 2 (3), 624-631 (2007).
- Palasek, S. A., Cox, Z. J., Collins, J. M. Limiting racemization and aspartimide formation in microwave-enhanced Fmoc solid phase peptide synthesis. J Pept Sci. 13 (3), 143-148 (2007).
- Murray, J. K., Aral, J., Miranda, L. P. Solid-Phase Peptide Synthesis Using Microwave Irradiation. Methods Mol. Biol. 716, 73-88 (2011).
- Galanis, A. S., Albericio, F., Grotli, M. Solid-Phase Peptide Synthesis in Water Using Microwave-Assisted Heating. Organic Letters. 11 (20), 4488-4491 (2009).
- Rizzolo, F., Sabatino, G., Chelli, M., Rovero, P., Papini, A. M. A convenient microwave-enhanced solid-phase synthesis of difficult peptide sequences: Case study of Gramicidin A and CSF114(Glc). Int. J. Pept. Res. Ther. 13 (1-2), 203-208 (2007).
- Matsushita, T., Hinou, H., Kurogochi, M., Shimizu, H., Nishimura, S. Rapid microwave-assisted solid-phase glycopeptide synthesis. Org Lett. 7 (5), 877-880 (2005).
- Nagaike, F., et al. Efficient microwave-assisted tandem N- to S-acyl transfer and thioester exchange for the preparation of a glycosylated peptide thioester. Org Lett. 8 (20), 4465-4468 (2006).
- Naruchi, K., et al. Construction and structural characterization of versatile lactosaminoglycan-related compound library for the synthesis of complex glycopeptides and glycosphingolipids. J. Org. Chem. 71 (26), 9609-9621 (2006).
- Brandt, M., Gammeltoft, S., Jensen, K. J. Microwave heating for solid-phase peptide synthesis: General evaluation and application to 15-mer phosphopeptides. Int. J. Pept. Res. Ther. 12 (4), 349-357 (2006).
- Harris, P. W. R., Williams, G. M., Shepherd, P., Brimble, M. A. The Synthesis of Phosphopeptides Using Microwave-assisted Solid Phase Peptide Synthesis. Int. J. Pept. Res. Ther. 14 (4), 387-392 (2008).
- Qvit, N. Microwave-assisted Synthesis of Cyclic Phosphopeptide on Solid Support. Chem. Biol. Drug Des. 85 (3), 300-305 (2014).
- Kato, D., Verhelst, S. H., Sexton, K. B., Bogyo, M. A general solid phase method for the preparation of diverse azapeptide probes directed against cysteine proteases. Org Lett. 7 (25), 5649-5652 (2005).
- Olivos, H. J., Alluri, P. G., Reddy, M. M., Salony, D., Kodadek, T. Microwave-assisted solid-phase synthesis of peptoids. Org Lett. 4 (23), 4057-4059 (2002).
- Gorske, B. C., Jewell, S. A., Guerard, E. J., Blackwell, H. E. Expedient synthesis and design strategies for new peptoid construction. Org Lett. 7 (8), 1521-1524 (2005).
- Grieco, P., et al. Design and microwave-assisted synthesis of novel macrocyclic peptides active at melanocortin receptors: discovery of potent and selective hMC5R receptor antagonists. J. Med. Chem. 51 (9), 2701-2707 (2008).
- Boutard, N., Jamieson, A. G., Ong, H., Lubell, W. D. Structure-Activity Analysis of the Growth Hormone Secretagogue GHRP-6 by alpha- and beta-Amino gamma-Lactam Positional Scanning. Chem. Biol. Drug Des. 75 (1), 40-50 (2010).
- Jamieson, A. G., et al. Positional scanning for peptide secondary structure by systematic solid-phase synthesis of amino lactam peptides. J. Am. Chem. Soc. 131 (22), 7917-7927 (2009).
- Hossain, M. A., Bathgate, R. A. D., Tregear, G., Wade, J. D. De Novo Design and Synthesis of Cyclic and Linear Peptides to Mimic the Binding Cassette of Human Relaxin. Annals of the New York Academy of Sciences. 1160, 16-19 (2009).
- Fowler, S. A., Stacy, D. M., Blackwell, H. E. Design and synthesis of macrocyclic peptomers as mimics of a quorum sensing signal from Staphylococcus aureus. Org Lett. 10 (12), 2329-2332 (2008).
- Cemazar, M., Craik, D. J. Microwave-assisted Boc-solid phase peptide synthesis of cyclic cysteine-rich peptides. J Pept Sci. 14 (6), 683-689 (2008).
- Miles, S. M., Leatherbarrow, R. J., Marsden, S. P., Coates, W. J. Synthesis and bio-assay of RCM-derived Bowman-Birk inhibitor analogues. Org Biomol Chem. 2 (3), 281-283 (2004).
- Murray, J. K., et al. Efficient synthesis of a beta-peptide combinatorial library with microwave irradiation. J. Am. Chem. Soc. 127 (38), 13271-13280 (2005).
- Churchill, E. N., Qvit, N., Mochly-Rosen, D. Rationally designed peptide regulators of protein kinase. C. Trends Endocrinol. Metab. 20 (1), 25-33 (2009).
- Mochly-Rosen, D., Qvit, N. Peptide inhibitors of protein-protein interactions. chimica Oggi / CHEMISTRY today. 28 (1), 14-16 (2010).
- Qvit, N., Mochly-Rosen, D. Highly specific modulators of protein kinase C localization: applications to heart failure. Drug Discov. Today Dis. Mech. 7 (2), e87-e93 (2010).
- Mougneau, E., et al. Expression cloning of a protective Leishmania antigen. Science. 268 (5210), 563-566 (1995).
- Kelly, B. L., Stetson, D. B., Locksley, R. M. Leishmania major LACK antigen is required for efficient vertebrate parasitization. J. Exp. Med. 198 (11), 1689-1698 (2003).
- Choudhury, K., et al. Trypanosomatid RACK1 orthologs show functional differences associated with translation despite similar roles in Leishmania pathogenesis. PLoS One. 6 (6), e20710 (2011).
- Gonzalez-Aseguinolaza, G., Taladriz, S., Marquet, A., Larraga, V. Molecular cloning, cell localization and binding affinity to DNA replication proteins of the p36/LACK protective antigen from Leishmania infantum. Eur. J. Biochem. 259 (3), 909-916 (1999).
- Gump, J. M., Dowdy, S. F. TAT transduction: the molecular mechanism and therapeutic prospects. Trends Mol. Med. 13 (10), 443-448 (2007).
- Aletras, A., Barlos, K., Gatos, D., Koutsogianni, S., Mamos, P. Preparation of the very acid-sensitive Fmoc-Lys(Mtt)-OH. Application in the synthesis of side-chain to side-chain cyclic peptides and oligolysine cores suitable for the solid-phase assembly of MAPs and TASPs. Int. J. Pept. Protein Res. 45 (5), 488-496 (1995).
- Li, D., Elbert, D. L. The kinetics of the removal of the N-methyltrityl (Mtt) group during the synthesis of branched peptides. J. Pept. Res. 60 (5), 300-303 (2002).
- Bourel, L., Carion, O., Gras-Masse, H., Melnyk, O. The deprotection of Lys(Mtt) revisited. J Pept Sci. 6 (6), 264-270 (2000).
- Tran, H., Gael, S. L., Connolly, M. D., Zuckermann, R. N. Solid-phase submonomer synthesis of peptoid polymers and their self-assembly into highly-ordered nanosheets. J Vis Exp. (57), e3373 (2011).
- Kaiser, E., Colescot, R. L., Bossinge, C. D., Cook, P. I. Color Test for Detection of Free Terminal Amino Groups in Solid-Phase Synthesis of Peptides. Anal. Biochem. 34 (2), 595-598 (1970).
- Christensen, T. Qualitative Test for Monitoring Coupling Completeness in Solid-Phase Peptide-Synthesis Using Chloranil. Acta Chem. Scand. Ser.B-Org. Chem. Biochem. 33 (10), 763-766 (1979).
- Qvit, N., Crapster, J. A. Peptides that Target Protein-Protein Interactions as an Anti-Parasite Strategy. chimica Oggi / CHEMISTRY today. 32 (6), 62-66 (2014).
- Byk, G., et al. Synthesis and biological activity of NK-1 selective, N-backbone cyclic analogs of the C-terminal hexapeptide of substance P. J. Med. Chem. 39 (16), 3174-3178 (1996).
- King, D. S., Fields, C. G., Fields, G. B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int. J. Pept. Protein Res. 36 (3), 255-266 (1990).
- Pedersen, S. L., Tofteng, A. P., Malik, L., Jensen, K. J. Microwave heating in solid-phase peptide synthesis. Chemical Society Reviews. 41 (5), 1826-1844 (2012).
- Colangelo, A. M., et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J. Neurosci. 28 (11), 2698-2709 (2008).
- Mesfin, F. B., Andersen, T. T., Jacobson, H. I., Zhu, S., Bennett, J. A. Development of a synthetic cyclized peptide derived from alpha-fetoprotein that prevents the growth of human breast cancer. J. Pept. Res. 58 (3), 246-256 (2001).
- Mizejewski, G. J., Muehlemann, M., Dauphinee, M. Update of alpha fetoprotein growth-inhibitory peptides as biotherapeutic agents for tumor growth and metastasis. Chemotherapy. 52 (2), 83-90 (2006).

