Induction of Myocardial Infarction in Adult Zebrafish Using Cryoinjury
Summary
Zebrafish represents a valuable model to study the mechanisms of heart regeneration in vertebrates. Here, we present a protocol for induction of a heart infarct in adult zebrafish using cryoinjury. This method results in massive cell death within 20% of the ventricular wall, similar to that observed in mammalian infarcts.
Abstract
The mammalian heart is incapable of significant regeneration following an acute injury such as myocardial infarction1. By contrast, urodele amphibians and teleost fish retain a remarkable capacity for cardiac regeneration with little or no scarring throughout life2,3. It is not known why only some non-mammalian vertebrates can recreate a complete organ from remnant tissues4,5. To understand the molecular and cellular differences between regenerative responses in different species, we need to use similar approaches for inducing acute injuries.
In mammals, the most frequently used model to study cardiac repair has been acute ischemia after a ligation of the coronary artery or tissue destruction after cryoinjury6,7. The cardiac regeneration in newts and zebrafish has been predominantly studied after a partial resection of the ventricular apex2,3. Recently, several groups have established the cryoinjury technique in adult zebrafish8-10. This method has a great potential because it allows a comparative discussion of the results obtained from the mammalian and non-mammalian species.
Here, we present a method to induce a reproducible disc-shaped infarct of the zebrafish ventricle by cryoinjury. This injury model is based on rapid freezing-thawing tissue, which results in massive cell death of about 20% of cardiomyocytes of the ventricular wall. First, a small incision was made through the chest with iridectomy scissors to access the heart. The ventricular wall was directly frozen by applying for 23-25 seconds a stainless steel cryoprobe precooled in liquid nitrogen. To stop the freezing of the heart, fish water at room temperature was dropped on the tip of the cryoprobe. The procedure is well tolerated by animals, with a survival rate of 95%.
To characterize the regenerative process, the hearts were collected and fixed at different days after cryoinjury. Subsequently, the specimen were embedded for cryosectioning. The slides with sections were processed for histological analysis, in situ hybridization and immunofluorescence. This undertaking enhances our understanding of the factors that are required for the regenerative plasticity in the zebrafish, and provide new insights into the machinery of cardiac regeneration. A conceptual and molecular understanding of heart regeneration in zebrafish will impact both developmental biology and regenerative medicine.
Protocol
1. Equipment Set-up
- The main tool used to perform cryoinjury is the cryoprobe, the pen-like instrument that is made of stainless steel (Figure 1A). The cylindrical handle is 40 mm long and has a diameter of 8 mm. The tip of the applicator is 6 mm long and 0.8 mm of diameter. The junction between the tip and the handle is conical 4 mm long. In addition, the handle of the cryoprobe should be isolated with a plastic tube and tape to avoid frostbite of the fingers while holding the tool during the procedure.
- Other materials that are needed for the cryosurgery are a stereomicroscope, sharp forceps, micro dissecting spring scissors and a plastic transfer pipette. In addition prepare a beaker for anesthetizing the fish and a plastic spoon for transferring the fish.
- During the surgery, the fish are placed on a moist sponge with its ventral side up (Figure 1B). To hold the animal in a stable procedure, an appropriate groove should be manually cut in the sponge.
- Prepare a tank with system water to transfer the fish after the surgery.
- Set up a double timer first with a 10 second countdown and then automatically a 24 second countdown.
2. The Cryoinjury Procedure
- Cool down the cryoprobe by immersing the tip in 3-5 cm of liquid nitrogen for at least 3 min.
- Anesthetize an adult zebrafish by immerging it in 0.02% tricaine until the fish turns on its back and its gills almost stop moving (approximately 90 seconds).
- Transfer the fish with a plastic spoon to a moist sponge that has been cut to hold a fish upside down (Figure 1B). Hold fish steady with forceps using non-dominant hand. Visually locate the posterior medial margin of the heart and use straight iridectomy scissors to puncture the skin. (Figure 1C).
- Make a small (approx. 2 mm) incision above the heart by cutting straight anteriorly through the skin, muscles (Figure 1D). Do not cut through the bony branchial apparatus, as this will kill the animal.
- Gently tear the silvery epithelial layer of the hypodermis (Figure 1E) with the tip of the scissors to have a direct access to the beating ventricle. Do not insert the scissors deeper in the body cavity, as this will puncture the heart. The beating heart should be well visible, and no extensive bleeding should occur during the thoracotomy (Figure 1F).
- Start the programmed timer that has been set for 10 seconds followed by 24 seconds. During 10 seconds take out the cryoprobe from the liquid nitrogen. Make sure that there is no more nitrogen on the cryoprobe by shaking it gently. Spread the incision laterally using forceps to open the chest (Figure 1F).
- Once the timer rings 10 seconds, touch immediately but gently the ventricle with the tip of the pre-cooled cryoprobe (Figure 1G).
- Once the timer rings 24 seconds, pour 2-3 mL of system water using a plastic pipette onto the chest to release the cryoprobe from tissue, and transfer the fish into the tank with system water.
- The fish should restart breathing after a few seconds, and then it should resume swimming. If it doesn’t breathe after around 45 seconds, stimulate the animal by squirting water into the gills with a plastic pipette until it starts breathing by itself. In our experience, 95% of the fish survive surgery, and all deaths occur on the day of surgery. It will not be necessary to suture incisions.
3. Heart Collection and Fixation
- Prepare 1 mL of 2% formalin in a microcentrifuge tube for fixing the heart, two forceps, micro-dissecting scissors and the moist slotted sponge.
- At the selected day after cryoinjury, euthanize the fish in 0.1% tricaine for 5 minutes.
- Place the fish ventral side up into a moist, slotted sponge. Make a large (approx. 4 mm) incision above the heart through the branchial cartilage with the micro dissecting scissors. Open widely the incision with forceps (Figure 1H).
- Pinch off the bulbus arteriosus, a white structure anterior to the ventricle (Figure 1 H-I), and remove the heart from the cavity by pulling it. A whole dissected heart is shown in Figure 1J and Figure 2A.
- Place up to 3 hearts into a microcentrifuge tube with 1 ml of 2% formalin. Gently turn the tube several times and keep it overnight at 4 °C.
4. Heart Mounting
- Rinse the hearts in PBS for 5 minutes. Transfer the hearts into 10 mL of 30% sucrose that should be pre-cooled at 4 °C, and mix gently. The specimen should initially float at the surface of sucrose solution. Continue incubation for 1 hour 20 minutes at 4 °C. After this time, the hearts will sink to the bottom of the tube.
- Prepare a box with dry ice. Take an embedding mold, and pour a 5 mm layer of O.C.T. mounting medium at the bottom of the mold.
- With forceps place the heart into the O. C. T mounting medium in the mold. Under the stereomicroscope, adjust the orientation of the specimen to achieve the ventricular apex on the bottom of the mold, and the bulbus arteriosus towards the top.
- Place the molds with specimen on dry ice. When the mounting medium begins to freeze, fill up the rest of the mold with O.C.T. medium and let it freeze completely. Keep the mold for at least 1 h at -80 °C before sectioning. The frozen specimen can be stored for many months at -80 °C.
5. Hearts Sectioning
- Set up a cryostat with a cutting size of 16 μm, a chamber temperature of -24 °C and the temperature of the specimen at -22 °C.
- Place the frozen block with the specimen in the cryostat and fix its orientation to begin cutting parallel to the bottom of the block.
- Prepare six Superfrost-plus slides per one block and numerate them from 1 to 6. Start cutting until the tissue is reached, and trim the block around the specimen with a razorblade.
- To obtain 6 replicates of one heart, take up the first section on slide 1, the second section on slide 2, the third on slide 3, etc. Once you placed the sixth section on slide 6, restart with slide 1 and continue until the whole organ is cut. Collect two rows of around 8 sections on the slide (Figure 3).
- Let the slides dry for 1 hour at room temperature. Store them for up to 1 year in tightly closed boxes at -20 °C.
6. Representative Results
Representative cardiac injury following this protocol is shown in dissected whole hearts at 4 dpci (days post cryoinjury; Figure 2D-F). To visualize the myocardial tissue in vivo, we used transgenic fish expressing EGFP and nuclear DsRed2 under the control of cardiac-specific cmlc-2 promoter11,12. The absence of EGFP and DsRed2 fluorescent signals demarcated a disc-shaped infarct zone along the apical-lateral ventricular wall.
To perform cellular and molecular analyses of the tissue, the hearts were fixed and sectioned with a cryostat. A representative slide with a consecutive series of transversal heart sections is shown in Figure 3.
The sections can be analyzed with various methods (Figure 4). To determine the extent of heart regeneration versus scarring, we performed histological analysis using Acid Fuchsin Orange-G (AFOG) staining, which differentially labels cardiac and fibrotic tissues9. The gene expression analyses were achieved according to the in situ hybridization procedure13. To detect the distribution of marker proteins, we applied immunofluorescence assay with specific antibodies9. A combination of diverse staining procedures leads to the identification of molecular and cellular mechanisms involved in cardiac regeneration following cryoinjury.
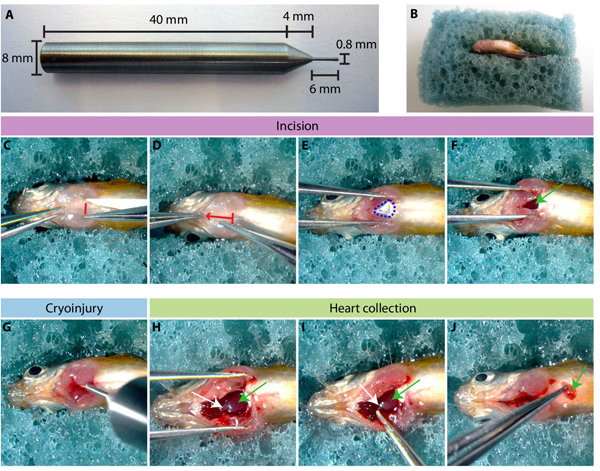
Figure 1. Inducing cryoinjury of the ventricle in the adult zebrafish. (A) A photograph of the cryoprobe. (B) An adult zebrafish is placed in the slit of the sponge with its ventral side up. (C-F) Chest incision to access the heart. (C) A small cut through the skin and muscles is made between the two pectoral fins (red line). (D) The scissors are inserted into the incision to cut the skin following the red arrow. (E) Underneath the skin, the fine layer of silvery hypodermis (encircled by blue dashed line) is gently opened to access the heart. (F) The incision is spread with the forceps and the beating ventricle (green arrow) is accessible for cryoinjury. (G) The cold cryoprobe is gently inserted into the chest to touch the heart. (H-J) Heart collection. (H) A deep and long incision is made through the branchial arches of the chest to access the pericardial cavity. Two heart structures are visible: bulbus arteriosus (white arrow) and ventricle (green arrow). (I) The bulbus arteriosus is hold with forceps and pulled out from the body cavity. (J) The entire heart is excised from of the body.
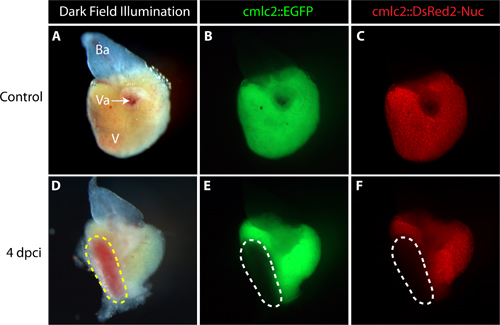
Figure 2. Representative control and cryoinjured hearts dissected from adult transgenic zebrafish expressing EGFP and nuclear DsRed2 in cardiomyocytes. (A-C) Uninjured heart. (A) The dark field illumination shows the ventricle (V), bulbus arteriosus (Ba, white) and the valves (Va), where the atrium was linked to the ventricle. (B and C) Fluorescent images of the intact ventricle display EGFP and DsRed2 expression in cardiomyocytes. (D-F) Heart at 4 days post cryoinjury (4 dpci) (D) The dark field illumination reveals a disc-shaped infarct (yellow dashed line). (E-F) Cardiomyocyte markers EGFP and DsRed2 are not detected in the infarct area, indicating the damaged myocardium (encircled by dashed line).
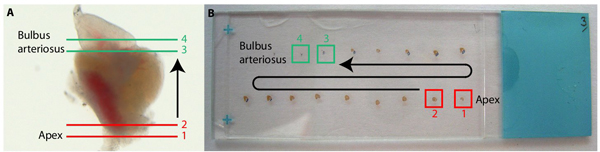
Figure 3. Sectioning of the heart. (A) Whole heart with the drawing of transversal sections starting from the bottom of the heart (1 and 2, in red) towards the top of the heart (3 and 4 in green). (B) Photography of a slide with the series of consecutive transversal sections stained with AFOG (Acid Fuchsin Orange-G). The first sections (1 and 2, red squares) contain the ventricular apex, and the last sections of the heart (3 and 4, green squares) comprise bulbus arteriosus.
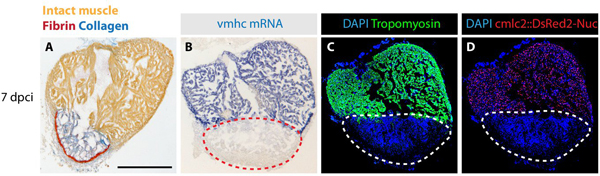
Figure 4. Examples of analysis performed on heart sections at 7 days post cryoinjury. (A) AFOG (Acid Fuchsin Orange-G) staining labels healthy muscle cells in orange, the scar tissue containing collagen in blue and fibrin in red. (B) In situ hybridization of ventricular myosin heavy chain (vmhc) mRNA visualizes the intact myocardium (blue staining). The injured tissue is devoid of the cardiac gene transcript (red dashed line). (C) Immunoassaying of Tropomyosin (green) labels intact cardiomyocytes and not damaged tissue (encircled with the dashed line). DAPI (blue) stains the nuclei of all the cells. (D) Genetically labeled cardiomyocytes express DsRed2 (red) in their nuclei. DAPI (blue) labels all the nuclei. The infarct zone does not contain DsRed2-positive cells (white dashed line).
Discussion
Cryoinjury is defined as the controlled damage of tissue by the precise application of extreme cold14. Direct thermal shock destroys the cells by protein destruction and by intracellular fluid ice crystal formation that ruptures the plasma membrane. Consequently, the injured cells die in the processes of apoptosis and necrosis14. Both of these cell death mechanisms have been shown to contribute to tissue loss after myocardial infarction15. Thus, cryoinjury represents a suitable model of inducing a heart infarct. Several studies reported this well proved method in various mammals, including mice, rats, rabbits and pigs6,7. The adaptation of the cryoinjury protocol in zebrafish enables a more comparative discussion on the infarct response between different species.
Two other groups independently developed the cryoinjury procedure for the adult zebrafish heart. The major differences are the surgery methods, the type of tools and applied freezing time. In the study by González-Rosa et al., the pericardium was opened by tearing the tissue instead of cutting. They used 0.3 mm copper filament linked to a polyamid tube pre-cooled in liquid nitrogen to freeze the heart for few seconds until thawing could be observed10. In the study by Schnabel et al., a conical piece of dry ice of 20 mm length with a pointed tip of 2 mm in its diameter was used to touch the heart for 10 seconds8. The two groups obtained ventricular damage through death of the myocardium. The advantage of our method is a more reproducible deposition of collagen-rich scar, which better mimics the early infarct healing responses observed in mammalian systems.
The critical step during the cryoinjury procedure in zebrafish is making an incision through the skin and the pericardial sac without puncturing the underlying heart. A correctly performed surgery causes little bleeding. Profuse bleeding indicates an unintentional puncture of the ventricle with the scissors. In such cases, the animals should be retracted from the experiment. In order to avoid puncturing the heart, the point of scissors should be aimed at a shallow angle to the skin.
Another important aspect for creating a reproducible injury is the exact positioning of the cold cryoprobe on the ventricle for the optimal precise duration. Too short freezing time will result in inflammation with little cell death. Prolonged freezing, on the other hand, may cause extensive damage of the heart, which may lead to enhanced mortality of the animals. We determined that the optimal time to hold the ventricle in the frozen state ranges between 23-26 seconds for a 2 cm long adult zebrafish, and it may differ dependently on the size of the animals.
Because our protocol is very simple and rapid, there are no experimental limitations in subjecting sufficient number of animals to obtain adequate biological replications. The cryosurgical treatment is very well tolerated by the animals. Collection of the hearts at different time points after cryoinjury allows detailed characterization of the subsequent phases during the regenerative process. The experimental analysis of the cryoinjured hearts may include 1) visualization of gene transcription by in situ hybridization, 2) detection of protein distribution by immunofluorescent staining, 3) histological imaging of different structures. Understanding the key healing processes after myocardial infarction in zebrafish will impact the field of regenerative biology. Moreover, it might be beneficial for designing novel therapeutic approaches in regenerative medicine.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank V. Zimmermann for excellent technical assistance and for fish care. This work was supported by the Swiss National Science Foundation, grant number: 310000_120611.
Materials
| Name of the reagent | Company | Catalogue number |
| Micro dissecting spring scissors | Roboz Surgical Instrument Co., Inc. | RS-5602 |
| Tricaine | Sigma-Aldrich | E10521 |
| Formaldehyde ~36% | Sigma-Aldrich | 47630 |
| Sucrose | Sigma-Aldrich | 84100 |
| Tissue-Tek O.C.T. compound | Sakura Finetek | 4583 |
| Slides Superfrost Plus | Fisher Scientific | 12-550-15 |
| Peel-A-Way Disposable Embedding Molds – Truncated Molds T8 | Polyscience, Inc. | 18985 |
References
- Laflamme, M. A., Murry, C. E. Heart regeneration. Nature. 473, 326-335 (2011).
- Singh, B. N., Koyano-Nakagawa, N., Garry, J. P., Weaver, C. V. Heart of newt: a recipe for regeneration. J. Cardiovasc. Transl. Res. 3, 397-409 (2010).
- Poss, K. D. Getting to the heart of regeneration in zebrafish. Semin. Cell Dev. Biol. 18, 36-45 (2007).
- Ausoni, S., Sartore, S. From fish to amphibians to mammals: in search of novel strategies to optimize cardiac regeneration. J. Cell Biol. 184, 357-364 (2009).
- Borchardt, T., Braun, T. Cardiovascular regeneration in non-mammalian model systems: what are the differences between newts and man. Thrombosis and haemostasis. 98, 311-318 (2007).
- Bos, E. J. v. a. n. d. e. n., Mees, B. M., de Waard, M. C., de Crom, R., Duncker, D. J. A novel model of cryoinjury-induced myocardial infarction in the mouse: a comparison with coronary artery ligation. Am. J. Physiol. Heart. Circ. Physiol. 289, H1291-H1300 (2005).
- van Amerongen, M. J., Harmsen, M. C., Petersen, A. H., Popa, E. R., van Luyn, M. J. Cryoinjury: a model of myocardial regeneration. Cardiovasc. Pathol. 17, 23-31 (2008).
- Schnabel, K., Wu, C. C., Kurth, T., Weidinger, G. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS ONE. 6, (2011).
- Chablais, F., Veit, J., Rainer, G., Jazwinska, A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev. Biol. 11, 21 (2011).
- González-Rosa, J. M., Martin, V., Peralta, M., Torres, M., Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development. 138, 1663-1674 (2011).
- Rottbauer, W. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell. 111, 661-672 (2002).
- Burns, C. G. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nature chemical biology. 1, 263-264 (2005).
- Chablais, F., Jazwinska, A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development. 137, 871-879 (2010).
- Baust, J. G., Gage, A. A. The molecular basis of cryosurgery. BJU Int. 95, 1187-1191 (2005).
- Whelan, R. S., Kaplinskiy, V., Kitsis, R. N. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annual review of physiology. 72, 19-44 (2010).

