中子自旋回声光谱学作为脂膜动力学和膜-蛋白相互作用的独特探针
Summary
本文介绍了中子自旋回声(NSE)脂质膜研究中的样品制备、数据减少和数据分析方案。脂质的明智标记使获得中等长度和时间尺度上的不同膜动力学,从而发生重要的生物过程。
Abstract
脂质双层体是细胞膜的主要基质,是营养交换、蛋白膜相互作用和病毒萌芽等重要细胞过程的主要平台。对于有效的生物活性,细胞膜应足够刚性,以保持细胞及其隔间的完整性,但流动性足以使膜成分(如蛋白质和功能领域)扩散和相互作用。这种弹性和流体膜特性的微妙平衡及其对生物功能的影响,需要更好地了解关键生物过程(例如膜变形和蛋白质结合事件)的中观长度和时间尺度上的集体膜动力学。能够有效探测这一动态范围的技术包括中子自旋回波(NSE)光谱学。结合二元标签,NSE可用于直接访问弯曲和厚度波动以及选择膜特征的中等动力学。本文简要介绍了NSE技术,概述了在脂膜上进行NSE实验的程序,包括样品制备和去子宫化方案的细节,以及数据收集和减少说明。本文还介绍了用于提取关键膜参数的数据分析方法,如弯曲刚性模态、区域可压缩模态和平面粘度。为了说明NSE研究的生物学重要性,探讨了NSE所探讨的膜现象的选例,即添加剂对膜弯曲刚性的影响、域形成对膜波动的影响以及膜蛋白相互作用的动态特征。
Introduction
在过去的几十年里,对细胞膜及其功能的理解有了显著的演变。以前认为细胞膜是定义细胞边界和家膜蛋白1的被动脂质双层蛋白,现在已逐渐转变为一种动态模型,其中脂质双层层在调节重要的生物过程(包括细胞信号、分子交换和蛋白质功能)方面发挥着重要作用,仅举几例2、3、4、5、6。认识到细胞膜具有高度动态性,不断进行改造和分子再分配,促使科学探索膜7、8、9的平衡结构。因此,已经开发了多种方法来研究生物和生物吸血脂膜中的各种动态模式。迄今为止,这些研究大多集中在扩散分子运动10,11,12,13和宏观形状波动14,15,16,留下一个显着的差距,了解中间膜动力学,即脂质组合体的集体波动,包括很少10-100的脂质分子。这些动态发生在几十到几100+的长度尺度上,以及随着时间尺度的子 n 到几百 ns(见图 1),这里称为中等尺度。事实上,正是在这些尺度上,关键的生物活动发生在膜17层。这包括病毒萌芽18,通道盖19,膜蛋白相互作用20。同样重要的是要指出,膜蛋白的能量景观21,22表明,蛋白质的构象变化–必要的调节作用–发生在23的集体膜波动的ns时间尺度,进一步强调间皮动力学在细胞膜的生物功能及其生物灵感类比20的重要性。本文重点研究脂质膜中两种主要的中观动态模式,即弯曲波动和厚度波动。
直接探测这些波动模式的主要挑战是难以同时使用标准光谱法访问其空间和时间尺度。另一个挑战是,直接接触技术可能会影响相同的波动,他们打算测量16。生物膜24、25的组成和结构复杂性进一步加剧了这种情况,导致非同质膜特征,包括脂质域形成26、27、28、29、30和膜不对称31、32、33要求选择性探针来了解不同膜特征的动态。幸运的是,这些挑战可以通过非侵入性中子光谱方法克服,如中子自旋回波(NSE),它固有地访问所需的长度和时间尺度,并进一步使选择性膜特征的研究不改变其物理化学环境34。事实上,在过去几年中,NSE光谱学已经发展成为一个独特而强大的集体膜动力学探测器35。NSE对脂质膜的研究结果对脂膜的机械36、37和粘性38、39特性有了新的认识,并揭示了脂膜在生物功能40、41中的潜在作用。
NSE光谱技术基于干涉测量仪器设计,最初由Mezei42提出,使用一系列自旋翻转器和磁线圈来控制中子自旋的预兆,因为中子穿过仪器。设计基于与样品位置有关磁场元素的磁反射(图1A)。这意味着,在中子和样品之间没有能量交换的情况下,中子在仪器的前半部分和后半部分(注意两个预切线圈之间的π翻转器)以相反的方向执行相同数量的自旋预演。”因此,中子的最终自旋状态相对于初始状态保持不变 – 一种称为自旋回声的现象(见图1A中的透明中子)。然而,当中子与样品进行能量相互作用时,能量交换会改变仪器后半部分的自旋前额,从而导致不同的最终自旋状态(见图1A)。这是实验性地检测为极化损失,稍后将在本文中显示。有关 NSE 技术的更多详细信息,读者将参考专用技术论文 42、43、44、45。
在此处,提供了简化的描述,以提供 NSE 可访问的长度和时间尺度的粗略估计。长度刻度由可实现的波维克转移范围(Q = 4 = sin = /é)决定,其中2 = 是散射角,+是中子波长。可以看到Q是由光谱仪第二臂的波长范围和旋转范围设置的(见图 1A)。NSE 光谱仪上典型的Q范围为 +0.02-2 =-146、47和高达 0.01-4 +-1,最近升级为 48,49,对应于 1-600 + 的空间尺度。另一方面,无障碍时间尺度是根据磁预切线圈内中子获得的总预切角(或相位)计算得出的,并且发现为50: 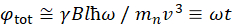 。在这个表达中,t是傅立中的时间定义为
。在这个表达中,t是傅立中的时间定义为 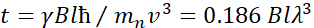 ,
, 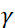 中子陀螺仪磁比在哪里,
中子陀螺仪磁比在哪里, 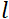 是线圈长度,
是线圈长度, 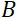 是线圈磁场的强度。值得指出的是,傅立叶时间是严格依赖于仪器几何、磁场强度和中子波长的数量。例如,使用波长 = 8 + 的中子
是线圈磁场的强度。值得指出的是,傅立叶时间是严格依赖于仪器几何、磁场强度和中子波长的数量。例如,使用波长 = 8 + 的中子 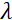 和
和 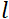 1.2 米和
1.2 米和 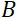 + 0.4 T 的仪器设置,Fourier 时间计算为t = 50 ns。在实验中,Fourier 时间通过改变预切线圈中的电流(即磁场强度)或使用不同的中子波长进行调整,从而产生典型的 NSE 时间尺度 =1 ps 到 100 ns。但是,最近 NSE 光谱仪的升级使访问时间更长的 Fourier 时间, 海因茨迈尔-莱布尼茨赞特鲁姆51号的 J-NSE-Phoenix 光谱仪和橡树岭国家实验室48号的 SNS-NSE 光谱仪高达 400 ns,以及劳兰格文学院 (ILL) 49 的 IN15 NSE 光谱仪上高达1,000ns。
+ 0.4 T 的仪器设置,Fourier 时间计算为t = 50 ns。在实验中,Fourier 时间通过改变预切线圈中的电流(即磁场强度)或使用不同的中子波长进行调整,从而产生典型的 NSE 时间尺度 =1 ps 到 100 ns。但是,最近 NSE 光谱仪的升级使访问时间更长的 Fourier 时间, 海因茨迈尔-莱布尼茨赞特鲁姆51号的 J-NSE-Phoenix 光谱仪和橡树岭国家实验室48号的 SNS-NSE 光谱仪高达 400 ns,以及劳兰格文学院 (ILL) 49 的 IN15 NSE 光谱仪上高达1,000ns。
除了直接访问膜动力学的长度和时间尺度外,NSE还具有中子同位素灵敏度的固有能力。具体来说,中子与氢同位素(生物系统中最丰富的元素)的相互作用能力,导致不同的中子散射长度密度,34或NSLD(相当于折射50的光学指数),当正二氧化硅被钚取代时。这支持一种称为对比度变异的方法,通常用于突出特定膜特征或隐藏其他特征–后一种方案称为对比匹配。对比变异/匹配的频繁应用是用水(NSLD = -0.56 × 10-6 +-2)用重水或D2O(NSLD = 6)代替水 .4 × 10-6 +-2) 以放大来自亲生脂质膜的中子信号 (NSLD = 0 × 10-6 +-2)。这种方法在膜结构研究中非常有效,因为D2O渗透到膜的头部群区域,可以准确确定膜厚度(见图2A,左面板)和不同脂质子组的位置,当应用更复杂的模型53,54。本文重点介绍了生物仿生膜中集体动力学研究的对比变异和选择膜特征的一些例子。
在这里,NSE 通过 NSE 对模型和生物相关脂质膜系统进行有形研究的有形示例,以脂质悬浮的形式,强调独立膜中的中尺度动力学,从而说明了 NSE 在提供对动态和功能膜特性的独特见解方面的有效性。对于NSE对平面膜动力学的测量,读者指的是关于放牧发生中子自旋回波光谱(GINSES)55,56和其他对齐多拉梅拉膜堆栈57,58,59,60的研究的专门出版物。
为了简单起眼,本文重点介绍了三种不同的膜去质方案,这些方案在经过充分研究的域形成中得到了说明, 或相分离,脂质双层系统1,2-二甲基-sn-甘油-3磷胆碱(DMPC)和1,2-脱脂-sn-甘油-3-磷胆碱(DSPC)混合物61,62。这两种脂质的特点是碳氢化合物链长度不匹配(DMPC 中的 14 碳/尾与 DSPC 中的 18 碳/尾)及其凝胶流体过渡温度(Tm、DMPC = 23 °C vs Tm、DSPC = 55 °C)。这导致在DMPC:DSPC膜的横向相分离在混合物63的上下过渡温度之间的温度。此处考虑的去质方案旨在演示 NSE 测量中对脂质膜的不同动态模式,即弯曲波动、厚度波动和横向域的选择性弯曲/厚度波动。所有脂质成分均报告为 DMPC:DSPC 双层,以 70:30 的摩尔分数为小数,使用 DMPC 和 DSPC 的商用亲生和渗透变体。所有样品制备步骤均基于 D2O 中的 4 mL 脂质悬浮液,脂质浓度为 50 毫克/mL,每个样品的总脂质为Mtot = 200 mg。
Protocol
Representative Results
Discussion
NSE 是一种功能强大且独特的技术,可测量不同条件下脂质膜的中等动力学。NSE 的有效利用取决于样品质量、中子对比度以及可用于特定样本的可访问动力学范围。因此,要成功进行 NSE 实验和收集高质量数据,需要几个关键步骤。确保在 NSE 实验中有效利用中子束时间的一个关键步骤是在 NSE 实验之前使用基于实验室的方法对脂质悬浮进行特征描述。对于外显子,挤出脂质体的大小分布(或扩散?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
阿什卡尔感谢长高,洛杉矶斯廷加丘和P.佐尔涅尔丘克进行了许多有益的讨论,并经常协助NSE在各自的光束线上进行实验。作者承认在NIST和ORNL使用中子自旋回波光谱仪。NIST 的 NSE 光谱仪由高分辨率中子散射中心提供支持,该中心是国家标准与技术研究所和国家科学基金会根据第 11 号协议建立的伙伴关系。DMR-1508249。ORNL 喷射中子源的 NSE 光谱仪由美国能源部基础能源科学办公室科学用户设施司提供支持。橡树岭国家实验室由美国国防部合同第一号公司 UT-Battelle 管理。DE-AC05-00OR22725。
Materials
| Chloroform (biotech grade) | Sigma Aldrich | 496189 | Biotech. grade, ≥99.8%, contains 0.5-1.0% ethanol as stabilizer |
| Circulating water bath | Julabo | SE-12 | Heating Circulator with smart pump, programmable temperature settings, and external sensor connection for measurement and control |
| Deuterium Oxide | Cambridge Isotopes Laboratories | DLM-4 | Deuterated water; Heavy water (D2O) (D, 99.9%) |
| Digital Semi-Microbalance | Mettler Toledo | MS105 | Semi-micro balance with 120 g capacity, 0.01 mg readability, high resolution weighing cell, ergonomic doors, and pipette-check application |
| Ethanol (molecular biology grade) | Sigma Aldrich | E7023 | 200 proof ethanol for molecular biology applications |
| Glass Pipets | VWR | 36360-536 | Disposable Soda Lime glass Pasteur pipets |
| Glass Vials | Thermo Scientific | B7990-1 | Borosilicate glass vials with PTFE/Silione septum caps |
| Lab grade freezer | Fisher Scientific | IU2886D | Ultra-low temprature freezer (-86 to -50 C) for long-term storage of lipids and proteins |
| Lipids (protaited or perdeuterated) | Avanti Polar Lipids | varies by lipid | Lipids can be purchased from Avanti in powder form or in a chloroform solution with the required amounts and deuteration schemes. |
| Millipore water purifier | Millipore Sigma | ZRQSVP3US | Direct-Q® 3 UV Water Purification System which deliver both pure and ultrapure water with a built-in UV lamp to reduce the levels of organics for biological applications |
| Mini Extruder Set | Avanti Polar Lipids | 610020 | Mini-extruder set includes mini-extruder, heating block, 2 GasTight Syringes, and 2 O-rings, Polycarbonate Membranes, and Filter Supports |
| Quick Connect Fittings | Grainger | 2YDA1 and 2YDA7 | Push-button tube fittings for QuickConnect water circulation applications, e.g. high temperature vesicle extrusion |
| Syringe Pump | SyringePump.com | New Era-1000 | Fully programmable syringe pump for infusion and withdrawal; programs up to 41 pumping phases with adjustable pumping rates, dispensed volumes, and extrusion cycles |
| Ultrasonic bath | Fisher Scientific | CPX2800 | Temperature controlled ultra sonic bath with programmable functionality for degassing and ultrasonic applications |
| Vacuum Oven | Thermo Scientific | 3608 | 0.7 cu ft vaccum oven with built-in-high-limit thermostat guards against overheating |
| Vortex Mixer | Fisher Scientific | 02-215-414 | Variable speed, analog control that allows low rpm start-up for gentle shaking or high-speed mixing for vigorous vortexing of samples |
References
- Singer, S. J., Nicolson, G. L. The fluid mosaic model of the structure of cell membranes. Science. 175 (4023), 720-731 (1972).
- Andersen, O. S., Koeppe, R. E. Bilayer thickness and membrane protein function: an energetic perspective. Annual Review of Biophysics and Biomolecular Structure. 36, 107-130 (2007).
- Lundbæk, J. A., Collingwood, S. A., Ingólfsson, H. I., Kapoor, R., Andersen, O. S. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. Journal of The Royal Society Interface. 7 (44), 373-395 (2010).
- Bradley, R. P., Radhakrishnan, R. Curvature-undulation coupling as a basis for curvature sensing and generation in bilayer membranes. Proceedings of the National Academy of Sciences of the United States of America. 113 (35), 117-124 (2016).
- Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A., Martinac, B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature. 418 (6901), 942-948 (2002).
- Jensen, M. &. #. 2. 1. 6. ;., Mouritsen, O. G. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1666 (1-2), 205-226 (2004).
- Rajendran, L., Simons, K. Lipid rafts and membrane dynamics. Journal of Cell Science. 118 (6), 1099-1102 (2005).
- Katchalsky, A., Spangler, R. Dynamics of membrane processes. Quarterly Reviews of Biophysics. 1 (2), 127-175 (1968).
- Rheinstädter, M. C. Collective molecular dynamics in proteins and membranes (Review). Biointerphases. 3 (2), 83-90 (2008).
- Fujiwara, T., Ritchie, K., Murakoshi, H., Jacobson, K., Kusumi, A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. The Journal of Cell Biology. 157 (6), 1071-1082 (2002).
- Hac, A. E., Seeger, H. M., Fidorra, M., Heimburg, T. Diffusion in two-component lipid membranes–a fluorescence correlation spectroscopy and monte carlo simulation study. Biophysical Journal. 88 (1), 317-333 (2005).
- Heinrich, M., Tian, A., Esposito, C., Baumgart, T. Dynamic sorting of lipids and proteins in membrane tubes with a moving phase boundary. Proceedings of the National Academy of Sciences of the United States of America. 107 (16), 7208-7213 (2010).
- Hormel, T. T., Kurihara, S. Q., Brennan, M. K., Wozniak, M. C., Parthasarathy, R. Measuring lipid membrane viscosity using rotational and translational probe diffusion. Physical Review Letters. 112 (18), 188101 (2014).
- Dimova, R. Recent developments in the field of bending rigidity measurements on membranes. Advances in Colloid and Interface Science. 208, 225-234 (2014).
- Bassereau, P., Sorre, B., Lévy, A. Bending lipid membranes: Experiments after W. Helfrich’s model. Advances in Colloid and Interface Science. 208, 47-57 (2014).
- Monzel, C., Sengupta, K. Measuring shape fluctuations in biological membranes. Journal of Physics D: Applied Physics. 49 (24), 243002 (2016).
- Deserno, M. Mesoscopic membrane physics: concepts, simulations, and selected applications. Macromolecular Rapid Communications. 30 (9-10), 752-771 (2009).
- Reynwar, B. J., et al. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature. 447 (7143), 461-464 (2007).
- Haswell, E. S., Phillips, R., Rees, D. C. Mechanosensitive channels: what can they do and how do they do it. Structure. 19 (10), 1356-1369 (2011).
- Phillips, R., Ursell, T., Wiggins, P., Sens, P. Emerging roles for lipids in shaping membrane-protein function. Nature. 459 (7245), 379-385 (2009).
- Dill, K. A., Chan, H. S. From Levinthal to pathways to funnels. Nature Structural Biology. 4 (1), 10-19 (1997).
- Henzler-Wildman, K., Kern, D. Dynamic personalities of proteins. Nature. 450 (7172), 964-972 (2007).
- Grimaldo, M., Roosen-Runge, F., Zhang, F., Schreiber, F., Seydel, T. Dynamics of proteins in solution. Quarterly Reviews of Biophysics. 52, 7 (2019).
- Lyman, E., Hsieh, C. -. L., Eggeling, C. From dynamics to membrane organization: experimental breakthroughs occasion a “modeling manifesto”. Biophysical Journal. 115 (4), 595-604 (2018).
- Arriaga, L. R., et al. Dissipative curvature fluctuations in bilayer vesicles: Coexistence of pure-bending and hybrid curvature-compression modes. The European Physical Journal. E, Soft Matter. 31 (1), 105-113 (2010).
- Honerkamp-Smith, A. R., Veatch, S. L., Keller, S. L. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1788 (1), 53-63 (2009).
- Veatch, S. L., Keller, S. L. Organization in lipid membranes containing cholesterol. Physical Review Letters. 89 (26), 268101 (2002).
- Heberle, F. A., et al. Bilayer thickness mismatch controls domain size in model membranes. Journal of the American Chemical Society. 135 (18), 6853-6859 (2013).
- Nickels, J. D., et al. The in vivo structure of biological membranes and evidence for lipid domains. PLOS Biology. 15 (5), 2002214 (2017).
- Simons, K., Ikonen, E. Functional rafts in cell membranes. Nature. 387 (6633), 569-572 (1997).
- van Meer, G., Voelker, D. R., Feigenson, G. W. Membrane lipids: where they are and how they behave. Nature Reviews. Molecular Cell Biology. 9 (2), 112-124 (2008).
- Liu, S. -. L., et al. Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nature Chemical Biology. 13, 268 (2016).
- Rothman, J., Lenard, J. Membrane asymmetry. Science. 195 (4280), 743-753 (1977).
- Ashkar, R., et al. Neutron scattering in the biological sciences: progress and prospects. Acta Crystallographica Section D. 74 (12), 1129-1168 (2018).
- Woodka, A. C., Butler, P. D., Porcar, L., Farago, B., Nagao, M. Lipid bilayers and membrane dynamics: insight into thickness fluctuations. Physical Review Letters. 109 (5), 058102 (2012).
- Chakraborty, S., et al. How cholesterol stiffens unsaturated lipid membranes. Proceedings of the National Academy of Sciences of the United States of America. 117 (36), 21896-21905 (2020).
- Arriaga, L. R., et al. Stiffening effect of cholesterol on disordered lipid phases: a combined neutron spin echo + dynamic light scattering analysis of the bending elasticity of large unilamellar vesicles. Biophysical Journal. 96 (9), 3629-3637 (2009).
- Nagao, M., Kelley, E. G., Ashkar, R., Bradbury, R., Butler, P. D. Probing elastic and viscous properties of phospholipid bilayers using neutron spin echo spectroscopy. The Journal of Physical Chemistry Letters. 8 (19), 4679-4684 (2017).
- Kelley, E. G., Butler, P. D., Ashkar, R., Bradbury, R., Nagao, M. Scaling relationships for the elastic moduli and viscosity of mixed lipid membranes. Proceedings of the National Academy of Sciences of the United States of America. 117 (38), 23365-23373 (2020).
- Rickeard, B. W., et al. Transverse lipid organization dictates bending fluctuations in model plasma membranes. Nanoscale. 12 (3), 1438-1447 (2020).
- Nickels, J. D., et al. Mechanical properties of nanoscopic lipid domains. Journal of the American Chemical Society. 137 (50), 15772-15780 (2015).
- Mezei, F. Neutron spin echo: A new concept in polarized thermal neutron techniques. Zeitschrift für Physik A Hadrons and Nuclei. 255 (2), 146-160 (1972).
- Hayter, J. B., Penfold, J. Neutron spin-echo integral transform spectroscopy. Zeitschrift für Physik B Condensed Matter. 35 (2), 199-205 (1979).
- Monkenbusch, M., Richter, D., Imae, T., Kanaya, T., Furusaka, M., Torikai, N. . Neutrons in Soft Matter. , 147-182 (2011).
- Pynn, R., Mezei, F., Pappas, C., Gutberlet, T. . Neutron Spin Echo. , 159-177 (2003).
- Holderer, O., et al. The JCNS neutron spin-echo spectrometer J-NSE at the FRM II. Measurement Science and Technology. 19 (3), 034022 (2008).
- Schleger, P., et al. The long-wavelength neutron spin-echo spectrometer IN15 at the Institut Laue-Langevin. Physica B: Condensed Matter. 241-243, 164-165 (1997).
- Holderer, O., Zolnierczuk, P., Pasini, S., Stingaciu, L., Monkenbusch, M. A better view through new glasses: Developments at the Jülich neutron spin echo spectrometers. Physica B: Condensed Matter. 562, 9-12 (2019).
- Farago, B., et al. The IN15 upgrade. Neutron News. 26 (3), 15-17 (2015).
- Ashkar, R. Selective dynamics in polymeric materials: Insights from quasi-elastic neutron scattering spectroscopy. Journal of Applied Physics. 127 (15), 151101 (2020).
- Pasini, S., Holderer, O., Kozielewski, T., Richter, D., Phoenix Monkenbusch, M. J-NSE- Phoenix, a neutron spin-echo spectrometer with optimized superconducting precession coils at the MLZ in Garching. Review of Scientific Instruments. 90 (4), 043107 (2019).
- Svergun, D. I., Koch, M. H. J., Timmins, P. A., May, R. P. . Small Angle X-Ray and Neutron Scattering from Solutions of Biological Macromolecules. , (2013).
- Eicher, B., et al. Joint small-angle X-ray and neutron scattering data analysis of asymmetric lipid vesicles. Journal of Applied Crystallography. 50 (2), 419-429 (2017).
- Heberle, F. A., et al. Model-based approaches for the determination of lipid bilayer structure from small-angle neutron and X-ray scattering data. European Biophysics Journal. 41 (10), 875-890 (2012).
- Jaksch, S., Koutsioubas, A., Mattauch, S., Holderer, O., Frielinghaus, H. Long-range excitations in phospholipid membranes. Chemistry and Physics of Lipids. 225, 104788 (2019).
- Jaksch, S., et al. Influence of ibuprofen on phospholipid membranes. Physical Review E. 91 (2), 022716 (2015).
- Armstrong, C. L., et al. Effect of cholesterol on the lateral nanoscale dynamics of fluid membranes. European Biophysics Journal. 41 (10), 901-913 (2012).
- Rheinstädter, M. C., Häußler, W., Salditt, T. Dispersion relation of lipid membrane shape fluctuations by neutron spin-echo spectrometry. Physical Review Letters. 97 (4), 048103 (2006).
- Armstrong, C. L., Häußler, W., Seydel, T., Katsaras, J., Rheinstädter, M. C. Nanosecond lipid dynamics in membranes containing cholesterol. Soft Matter. 10 (15), 2600-2611 (2014).
- Nickels, J. D., et al. Lipid rafts: buffers of cell membrane physical properties. The Journal of Physical Chemistry B. 123 (9), 2050-2056 (2019).
- Michonova-Alexova, E. I., Sugár, I. P. Component and state separation in DMPC/DSPC lipid bilayers: a Monte Carlo simulation study. Biophysical Journal. 83 (4), 1820-1833 (2002).
- Sugár, I. P., Thompson, T. E., Biltonen, R. L. Monte Carlo simulation of two-component bilayers: DMPC/DSPC mixtures. Biophysical Journal. 76 (4), 2099-2110 (1999).
- Mabrey, S., Sturtevant, J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proceedings of the National Academy of Sciences. 73 (11), 3862-3866 (1976).
- . Neutron activation and scattering calculator Available from: https://www.ncnr.nist.gov/resources/activation/ (2021)
- Scott, H. L., et al. On the mechanism of bilayer separation by extrusion, or why your LUVs are not really unilamellar. Biophysical Journal. 117 (8), 1381-1386 (2019).
- Ashkar, R., et al. Tuning membrane thickness fluctuations in model lipid bilayers. Biophysical Journal. 109 (1), 106-112 (2015).
- Carrillo, J. -. M. Y., Katsaras, J., Sumpter, B. G., Ashkar, R. A computational approach for modeling neutron scattering data from lipid bilayers. Journal of Chemical Theory and Computation. 13 (2), 916-925 (2017).
- Azuah, R. T. DAVE: a comprehensive software suite for the reduction, visualization, and analysis of low energy neutron spectroscopic data. Journal of Research of the National Institute of Standards and Technology. 114 (6), 341-358 (2009).
- Van Hove, L. Correlations in space and time and born approximation scattering in systems of interacting particles. Physical Review. 95 (1), 249-262 (1954).
- Zilman, A. G., Granek, R. Undulations and dynamic structure factor of membranes. Physical Review Letters. 77 (23), 4788-4791 (1996).
- Kelley, E. G., Butler, P. D., Nagao, M. . Collective dynamics in model biological membranes measured by neutron spin echo spectroscopy. , 131-176 (2019).
- Zheng, Y., Michihiro, N., Dobrin, P. B. Bending elasticity of saturated and monounsaturated phospholipid membranes studied by the neutron spin echo technique. Journal of Physics: Condensed Matter. 21 (15), 155104 (2009).
- Sharma, V. K., Qian, S. Effect of an antimicrobial peptide on lateral segregation of lipids: a structure and dynamics study by neutron scattering. Langmuir. 35 (11), 4152-4160 (2019).
- Boggara, M. B., Faraone, A., Krishnamoorti, R. Effect of pH and Ibuprofen on the Phospholipid Bilayer Bending Modulus. The Journal of Physical Chemistry B. 114 (24), 8061-8066 (2010).
- Lee, J. -. H., et al. Thermal fluctuation and elasticity of lipid vesicles interacting with pore-forming peptides. Physical Review Letters. 105 (3), 038101 (2010).
- Chakraborty, S., Abbasi, A., Bothun, G. D., Nagao, M., Kitchens, C. L. Phospholipid bilayer softening due to hydrophobic gold nanoparticle inclusions. Langmuir. 34 (44), 13416-13425 (2018).
- Hoffmann, I., et al. Softening of phospholipid membranes by the adhesion of silica nanoparticles – as seen by neutron spin-echo (NSE). Nanoscale. 6 (12), 6945-6952 (2014).
- Watson, M. C., Brown, F. L. H. Interpreting membrane scattering experiments at the mesoscale: the contribution of dissipation within the bilayer. Biophysical Journal. 98 (6), 9-11 (2010).
- Seifert, U., Langer, S. A. Viscous modes of fluid bilayer membranes. Europhysics Letters (EPL). 23 (1), 71-76 (1993).
- Bingham, R. J., Smye, S. W., Olmsted, P. D. Dynamics of an asymmetric bilayer lipid membrane in a viscous solvent. EPL (Europhysics Letters). 111 (1), 18004 (2015).
- Rawicz, W., Olbrich, K. C., McIntosh, T., Needham, D., Evans, E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophysical Journal. 79 (1), 328-339 (2000).
- Doktorova, M., LeVine, M. V., Khelashvili, G., Weinstein, H. A new computational method for membrane compressibility: bilayer mechanical thickness revisited. Biophysical Journal. 116 (3), 487-502 (2019).
- Evans, E., Needham, D. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion and colloidal interactions. The Journal of Physical Chemistry. 91 (16), 4219-4228 (1987).
- Lesieur, S., Grabielle-Madelmont, C., Paternostre, M. T., Ollivon, M. Size analysis and stability study of lipid vesicles by high-performance gel exclusion chromatography, turbidity, and dynamic light scattering. Analytical Biochemistry. 192 (2), 334-343 (1991).
- Heberle, F. A., et al. Direct label-free imaging of nanodomains in biomimetic and biological membranes by cryogenic electron microscopy. Proceedings of the National Academy of Sciences of the United States of America. 117 (33), 19943-19952 (2020).
- Cornell, C. E., Mileant, A., Thakkar, N., Lee, K. K., Keller, S. L. Direct imaging of liquid domains in membranes by cryo-electron tomography. Proceedings of the National Academy of Sciences of the United States of America. 117 (33), 19713-19719 (2020).
- Yao, X., Fan, X., Yan, N. Cryo-EM analysis of a membrane protein embedded in the liposome. Proceedings of the National Academy of Sciences of the United States of America. 117 (31), 18497-18503 (2020).
- Kučerka, N., Nieh, M. -. P., Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1808 (11), 2761-2771 (2011).
- Nielsen, J. E., Bjørnestad, V. A., Lund, R. Resolving the structural interactions between antimicrobial peptides and lipid membranes using small-angle scattering methods: the case of indolicidin. Soft Matter. 14 (43), 8750-8763 (2018).
- Kučerka, N., et al. Lipid bilayer structure determined by the simultaneous analysis of neutron and X-ray scattering data. Biophysical Journal. 95 (5), 2356-2367 (2008).
- Kelley, E. G., Butler, P. D., Nagao, M. Scaling of lipid membrane rigidity with domain area fraction. Soft Matter. 15 (13), 2762-2767 (2019).
- Brüning, B. -. A., et al. Bilayer undulation dynamics in unilamellar phospholipid vesicles: Effect of temperature, cholesterol and trehalose. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1838 (10), 2412-2419 (2014).
- Kučerka, N., et al. Areas of monounsaturated diacylphosphatidylcholines. Biophysical Journal. 97 (7), 1926-1932 (2009).
- Sharma, V. K., Mamontov, E., Anunciado, D. B., O’Neill, H., Urban, V. S. Effect of antimicrobial peptide on the dynamics of phosphocholine membrane: role of cholesterol and physical state of bilayer. Soft Matter. 11 (34), 6755-6767 (2015).
- Kelley, E. G., Butler, P. D., Nagao, M. Collective dynamics in lipid membranes containing transmembrane peptides. Soft Matter. , (2021).
- Yu, J., et al. Structure and dynamics of lipid membranes interacting with antivirulence end-phosphorylated polyethylene glycol block copolymers. Soft Matter. 16 (4), 983-989 (2020).
- Stingaciu, L. -. R., et al. Revealing the dynamics of thylakoid membranes in living cyanobacterial cells. Scientific Reports. 6 (1), 19627 (2016).
- Stingaciu, L. -. R., O’Neill, H. M., Liberton, M., Pakrasi, H. B., Urban, V. S. Influence of chemically disrupted photosynthesis on cyanobacterial thylakoid dynamics in synechocystis sp. PCC 6803. Scientific Reports. 9 (1), 5711 (2019).
- Miller, I. R. Energetics of fluctuation in lipid bilayer thickness. Biophysical Journal. 45 (3), 643-644 (1984).
- Nagao, M. Observation of local thickness fluctuations in surfactant membranes using neutron spin echo. Physical Review E. 80 (3), 031606 (2009).

