荧光显微镜用于人肿瘤细胞和肿瘤异种移植小鼠中巨细胞增多介导的ATP内化
Summary
我们开发了一种可重复的方法,以可视化具有高细胞分辨率的不可水解荧光三磷酸腺苷(ATP)的内化,ATP替代物。我们使用独立的 体外 和 体内 测定验证了我们的方法 – 人肿瘤细胞系和用人类肿瘤组织异种移植的免疫缺陷小鼠。
Abstract
三磷酸腺苷(ATP),包括细胞外ATP(eATP),已被证明在肿瘤发生的各个方面起重要作用,例如耐药性,上皮间充质转变(EMT)和转移。肿瘤内eATP的浓度比正常组织高103 至104 倍。虽然eATP作为激活嘌呤能信号传导的信使进行EMT诱导,但它也通过上调巨噬细胞增多症(一种特定类型的内吞作用)被癌细胞内化,以执行各种生物学功能。这些功能包括为需要ATP的生化反应提供能量,在信号转导期间捐赠磷酸基团,以及促进或加速作为转录辅因子的基因表达。ATP唾手可得,其在癌症等领域的研究无疑将会增加。然而,eATP研究仍处于早期阶段,在eATP和内化细胞内ATP所发挥的重要和多功能活动可以完全解开之前,尚未解决的问题仍未得到解答。
这些作者的实验室对这些早期eATP研究的贡献包括不可水解荧光ATP的显微成像,以及高分子量和低分子量荧光葡聚糖,其用作巨噬细胞增多和内吞作用示踪剂,以及各种内吞作用抑制剂,以监测和表征eATP内化过程。该成像方式应用于肿瘤细胞系和免疫缺陷小鼠,异种移植人癌症肿瘤, 以研究eATP在体外 和 体内的内化。本文描述了这些 体外 和 体内 方案,重点是修改和微调测定条件,以便可以在不同的系统中成功进行巨噬细胞增生/内吞作用介导的eATP内化测定。
Introduction
肿瘤内细胞外(即)营养素的机会性吸收最近被命名为癌症代谢的关键标志1。这些重要的营养素之一是ATP,因为IEATP的浓度比正常组织中发现的浓度高103和104倍,在几百μM到低mM 2,3,4,5的范围内。作为一种关键的能量和信号分子,ATP在癌细胞和健康细胞的细胞代谢中起着核心作用6,7,8。细胞外ATP不仅参与癌细胞生长,而且还促进耐药性9。最近已经确定了以前未被识别的ATP功能,例如亲水活性,因此涉及ATP参与阿尔茨海默氏症等疾病10。事实上,我们对ATP及其在癌细胞,健康细胞和其他患病细胞中的功能的理解似乎远未完成。然而,由于ATP在细胞中的不稳定性和高周转率,监测ATP穿过细胞膜并进入细胞的运动在技术上具有挑战性。
为了解决这个问题并满足该研究领域的需求,开发了一种方法,其中使用不可水解的荧光ATP(NHF-ATP)(图1)作为替代物,以可视化ATP的内化并观察内化ATP的细胞内空间定位,无论是在体外还是在体内11,12.NHF-ATP已被证明可以替代内源性ATP来研究ATP在动物细胞膜上的运动,无论是在癌细胞系中还是在免疫缺陷小鼠上异种移植的人肿瘤组织中11,12。此外,将巨噬细胞增多抑制剂施用于阻断eATP内化的细胞,表明eATP的细胞内摄取涉及巨噬细胞机制9,11,12。该协议允许针对细胞特异性蛋白质的免疫基colabel,从而鉴定哪种细胞类型内化NHF-ATP。使用体内肿瘤异种移植物和高分辨率显微镜,NHF-ATP可以在组织样品甚至单个细胞内的空间上可视化。这些方法还允许定量分析,例如细胞摄取的百分比,大卵子细胞囊泡的数量和内化动力学。本文详细描述了NHF-ATP如何单独或与内吞作用 – 示踪剂荧光葡聚糖13,14,15,16一起工作,可以在不同的实验环境中用于研究ATP的内化和细胞内定位,在细胞内化之后。
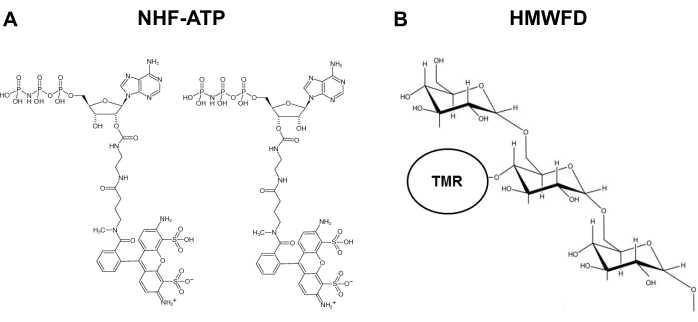
图1:不可水解荧光ATP和四甲基罗丹明标记的高分子量荧光葡聚糖的结构。(B) HMWFD的示意图。缩写: ATP = 三磷酸腺苷;NHF-ATP = 不可水解荧光 ATP;TMR = 四甲基罗丹明;HMWFD = 高分子量荧光葡聚糖。请点击此处查看此图的放大版本。
Protocol
Representative Results
Discussion
开发了一种用于不可水解ATP的细胞内化的空间,时间和定量分析方法。该方法广泛适用于各种生物系统,包括各种致瘤模型,我们为其提供技术指导和代表性数据。为了 在体内 ATP内化研究期间获得可解释的数据(方案第4节),限制从肿瘤内葡聚糖注射到冷冻包埋的实验时间至关重要。固定组织载玻片 – 肿瘤后切片 – 是荧光显微镜成像之前的必要步骤。这两个关键步骤共同确保肿瘤细胞在?…
Disclosures
The authors have nothing to disclose.
Acknowledgements
冷冻切除术在俄亥俄大学组织病理学核心现场进行。这项工作部分得到了创业基金(俄亥俄大学艺术与科学学院)对C Nielsen的支持;NIH授予R15 CA242177-01和RSAC奖给X Chen。
Materials
| A549 cells, human lung epithelial, carcinoma | National Cancer Institute | n/a | Less expensive source |
| Acetone | Fisher Scientific | S25904 | |
| Aluminum foil, Reynolds | Grainger | 6CHG6 | |
| Aqueous Mounting Medium, ProLong Gold Anti-fade Reagent | ThermoFisher | P36930 | |
| ATP analog | Jena Biosciences | NK-101 | |
| Autoclave, sterilizer | Grainger | 33ZZ40 | |
| Blades, cryostat, high profile | C. L. Sturkey, Inc. | DT554550 | |
| Calipers, vernier | Grainger | 4KU77 | |
| Cell medium, Ham's Nutrient Mixture F12, serum-free | Millipore Sigma | 51651C-1000ML | |
| Centrifuge, refrigerated with swinging bucket rotor | Eppendorf | 5810R | |
| Chloroform | Acros Organics | 423555000 | |
| Conical tube, 15 mL | VWR | 21008-216 | |
| Conical tube, 50 mL | VWR | 21008-242 | |
| Coverslips, glass, 12 mm | Corning | 2975-245 | |
| Cryostat, Leica CM1950 | Leica Biosystems | CM1950 | |
| Delicate task wipe, Kim Wipes | Kimberly-Clark | 34155 | |
| Dextran, Lysine fixable, High Molecular Weight (HMW) | Invitrogen | D1818 | MW = 70,000, Tetramethylrhodamine |
| Dextran, Neutral, High Molecular Weight (HMW) | Invitrogen | D1819 | |
| Dulbecco's Modified Eagle Medium (DMEM), serum-free | Fisher Scientific | 11885076 | |
| Dry ice | Local delivery | Custom order | |
| Epifluorescent imaging system, Nikon NiU and Nikon NIS Elements acquisition software | Nikon | Custom order | |
| Ethanol | Fisher Scientific | BP2818-4 | |
| Fetal bovine serum (FBS) | ThermoFisher | 16000044 | |
| Forceps, Dumont #7, curved | Fine Science Tools | 11274-20 | |
| Forceps, Dumont #5, straight | Fine Science Tools | 11254-20 | |
| Gloves (small, medium, large) | Microflex | N191, N192, N193 | |
| Gloves, MAPA Temp-Ice 700 Thermal (for handling dry ice) | Fisher Scientific | 19-046-563 | |
| Hemocytometer | Daigger | EF16034F EA | |
| Incubator, cell culture | Eppendorf | Galaxy 170 S | |
| Labelling tape | Fisher Scientific | 159015R | |
| Marking pen, Sharpie (ultra-fine) | Staples | 642736 | |
| Mice, immunodeficient (Nu/J) | Jackson Laboratory | 2019 | |
| Microcentrifuge, accuSpin Micro17 | Fisher Scientific | 13-100-675 | |
| Microcentrifgue tubes, Eppendorf tubes (1.5 mL) | Axygen | MCT-150-C | |
| Microscope slide box | Fisher Scientific | 50-751-4983 | |
| Needle, 27 gauge | Becton-Dickinson | 752 0071 | |
| Paintbrush | Grainger | 39AL12 | |
| Paper towels | Staples | 33550 | |
| Paraformaldehyde | Acros Organics | 416785000 | |
| Penicillin/Streptomycin | Gibco | 15140122 | |
| Perforated spoon, 15 mm diameter, 135 mm length | Roboz Surgical Instrument Co. | RS-6162 | |
| Phosphate buffered saline (PBS) | Fisher Scientific | BP3991 | |
| Pipet tips (10 μL) | Fisher Scientific | 02-707-438 | |
| Pipet tips (200 μL) | Fisher Scientific | 02-707-411 | |
| Pipet tips (1000 μL) | Fisher Scientific | 02-707-403 | |
| Pipets, serological (10 mL) | VWR | 89130-910 | |
| Pippetor, Gilson P2 | Daigger | EF9930A | |
| Pipettor Starter Kit, Gilson (2-10 μL, 20-200 μL, 200-1000 μL) | Daigger | EF9931A | |
| Platform shaker – orbital, benchtop | Cole-Parmer | EW-51710-23 | |
| Positively-charged microscope slides, Superfrost | Fisher Scientific | 12-550-15 | |
| Scalpel, size 10, Surgical Design, Inc. | Fisher Scientific | 22-079-707 | |
| Scissors, surgical – sharp, curved | Fine Science Tools | 14005-12 | |
| Software for image analysis, Nikon Elements | Nikon | Custom order | |
| Software for image analysis, ImageJ (FIJI) | National Institutes of Health | n/a | Download online (free) |
| Specimen disc 30 mm (chuck holder), cryostat accessory | Leica Biosystems | 14047740044 | |
| Staining tray, 245 mm BioAssay Dish | Corning | 431111 | |
| Syringe, 1 cc | Becton-Dickinson | 309623 | |
| Tape, laboratory, 19 mm width | Fisher Scientific | 15-901-5R | |
| Timer | Fisher Scientific | 14-649-17 | |
| Tissue culture dish, 100 x 15 mm diameter | Fisher Scientific | 08-757-100D | |
| Tissue culture flask, 225 cm2 | ThermoFisher | 159933 | |
| Tissue culture plate, 24-well | Becton-Dickinson | 353226 | |
| Tissue embedding mold, stainless steel | Tissue Tek | 4161 | |
| Tissue Freezing Medium, Optimal Cutting Temperature (OCT) | Fisher Scientific | 4585 | |
| Trypsin-EDTA (ethylenediaminetetraacetic acid), 0.25% | Gibco | 25200072 | |
| Water bath, Precision GP 2S | ThermoFisher | TSGP2S |
References
- Pavlova, N. N., Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metabolism. 23 (1), 27-47 (2016).
- Pellegatti, P., et al. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS ONE. 3, 25992008 (2008).
- Falzoni, S., Donvito, G., Di Virgilio, F. Detecting adenosine triphosphate in the pericellular space. Interface Focus. 3 (3), 20120101 (2013).
- Michaud, M., et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 334, 1573-1577 (2011).
- Wilhelm, K., et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nature Medicine. 16, 1434-1438 (2010).
- Vander Heiden, M. G., Cantley, L. C., Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 324, 1029-1033 (2009).
- Cairns, R. A., Harris, I. S., Mak, T. W. Regulation of cancer cell metabolism. Nature Reviews Cancer. 11, 85-95 (2011).
- Chen, X., Qian, Y., Wu, S. The Warburg effect: evolving interpretations of an established concept. Free Radical Biology & Medicine. 79, 253-263 (2015).
- Wang, X., et al. Extracellular ATP, as an energy and phosphorylating molecule, induces different types of drug resistances in cancer cells through ATP internalization and intracellular ATP level increase. Oncotarget. 8 (5), 87860-87877 (2017).
- Patel, A., et al. ATP as a biological hydrotrope. Science. 356, 753-756 (2017).
- Qian, Y., et al. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Letters. 351, 242-251 (2014).
- Qian, Y., Wang, X., Li, Y., Cao, Y., Chen, X. Extracellular ATP a new player in cancer metabolism: NSCLC cells internalize ATP in vitro and in vivo using multiple endocytic mechanisms. Molecular Cancer Research. 14, 1087-1096 (2016).
- Commisso, C., et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 497, 633-637 (2013).
- Li, L., et al. The effect of the size of fluorescent dextran on its endocytic pathway. Cell Biology International. 39, 531-539 (2015).
- Yanagawa, Y., Matsumoto, M., Togashi, H. Enhanced dendritic cell antigen uptake via alpha2 adrenoceptor-mediated PI3K activation following brief exposure to noradrenaline. Journal of Immunology. 185, 5762-5768 (2010).
- Hoppe, H. C., et al. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrobial Agents & Chemotherapy. 48, 2370-2378 (2004).
- Chaudry, I. H. Does ATP cross the cell plasma membrane. Yale Journal of Biology & Medicine. 55, 1-10 (1982).
- Pant, H. C., Terakawa, S., Yoshioka, T., Tasaki, I., Gainer, H. Evidence for the utilization of extracellular [gamma-32P]ATP for the phosphorylation of intracellular proteins in the squid giant axon. Biochimica et Biophysica Acta. 582, 107-114 (1979).
- Chaudry, I. H., Baue, A. E. Further evidence for ATP uptake by rat tissues. Biochimica et Biophysica Acta. 628, 336-342 (1980).
- Koppenol, W. H., Bounds, P. L., Dang, C. V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nature Reviews Cancer. 11, 325-337 (2011).
- Dang, C. V. Links between metabolism and cancer. Genes & Development. 26, 877-890 (2012).
- Israelsen, W. J., Vander Heiden, M. G. ATP consumption promotes cancer metabolism. Cell. 143, 669-671 (2010).
- Koster, J. C., Permutt, M. A., Nichols, C. G. Diabetes and insulin secretion: the ATP-sensitive K+ channel (K ATP) connection. Diabetes. 54, 3065-3072 (2005).
- Szendroedi, J., et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Medicine. 4, 154 (2007).
- Miyamoto, S., et al. Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine. 7, 121-134 (2016).

