通过显微注射在类 圆线虫 物种中产生转基因和敲除
Summary
寄生线虫 类圆线 虫粪类和大 鼠类圆线 虫的功能基因组工具包包括转基因、CRISPR/Cas9 介导的诱变和 RNAi。该协议将演示如何使用性腺内显微注射将转基因和CRISPR成分引入 粪类猪笼草 和 鼠李糖杆菌中。
Abstract
类圆线虫属由多种具有不同宿主范围的穿透皮肤线虫组成,包括粪类圆线虫和大鼠类圆线虫。S. stercoralis是一种人类寄生虫,穿透皮肤的线虫,感染约6.1亿人,而大鼠寄生虫S. ratti与粪类S. stercoralis密切相关,通常用作粪类菌丝虫的实验室模型。S. stercoralis和S. ratti都很容易通过性内显微注射的外源核酸递送技术产生转基因和敲除,因此,已经成为其他尚未适应该技术的寄生蠕虫的模型系统。
寄生 类圆线虫 成虫栖息在宿主的小肠中,并通过粪便将后代释放到环境中。一旦进入环境,幼虫就会发育成自由生活的成虫,它们生活在粪便中并产生必须找到并入侵新宿主的后代。这种环境生成是 类圆线虫 物种所独有的,在形态上与自由生活线虫 秀丽隐杆线虫 模型足够相似,为 秀丽隐杆线虫 开发的技术可以适应与这些寄生线虫一起使用,包括性腺内显微注射。使用性腺内显微注射,可以将各种各样的转基因引入类 圆线虫中。CRISPR / Cas9组分也可以显微注射以产生突变 的圆线虫 幼虫。这里,描述了性腺内显微注射到 类圆线虫的技术, 包括自由生活成人的制备,注射程序和转基因后代的选择。包括使用CRISPR / Cas9诱变产生的转基因 类圆线虫 幼虫的图像。本文的目的是使其他研究人员能够使用显微注射来产生转基因和突变 的类圆线虫。
Introduction
与更广泛认可的钩虫和蛔虫蛔虫相比,类粪类圆线虫长期以来一直被忽视为一种重要的人类病原体。以前对蠕虫负担的研究往往严重低估了粪类链球菌的患病率,因为粪类疟原虫的常见诊断方法的敏感性较低 2.近年来,基于改进的诊断工具的流行病学研究估计,粪类菌感染的真实患病率远高于先前报告的,全世界约有6.1亿人2。
S. stercoralis和其他类圆线虫物种,包括密切相关的大鼠寄生虫和常见的实验室模型S. ratti,都有一个不寻常的生命周期,对实验基因组研究有利,因为它由寄生和自由生活(环境)第3代组成(图1)。具体来说,S. stercoralis和S. ratti都可以通过单一的自由生活世代循环。自由生活的一代由后寄生虫幼虫组成,这些幼虫发育成自由生活的成年雄性和雌性;自由生活的成虫的所有后代都发育成感染性幼虫,必须感染宿主才能继续生命周期。此外,这种环境或自由生活的一代可以在实验室中通过实验操纵。由于自由生活的类圆线虫成虫和秀丽隐杆线虫成虫具有相似的形态,因此最初为秀丽隐杆线虫开发的性腺内显微注射等技术可以适用于自由生活的成年类圆线虫4,5。虽然DNA通常被引入自由生活的成年雌性中,但类圆线虫的雄性和雌性都可以被显微注射6。因此,功能基因组工具可用于询问类圆线虫生物学的许多方面。其他寄生线虫缺乏自由生活的一代,因此,不容易适应功能基因组技术3。
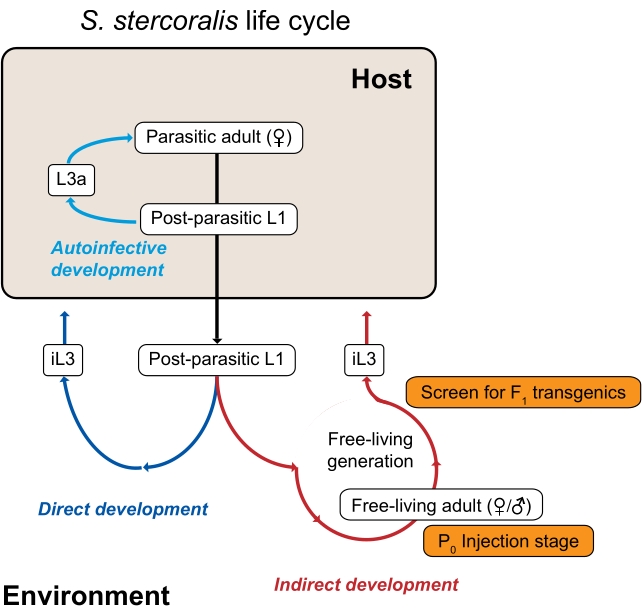
图1:类圆线虫的粪类动物生命周期。 粪类孢子虫寄生雌性栖息在其哺乳动物宿主(人类,非人类灵长类动物,狗)的小肠中。寄生雌性通过单性生殖繁殖,并在小肠内产卵。卵在宿主体内孵化成后寄生幼虫,然后与粪便一起进入环境。如果寄生后幼虫是雄性,它们会发育成自由生活的成年雄性。如果寄生后幼虫是雌性,它们可以发育成自由生活的成年雌性(间接发育)或第三阶段感染性幼虫(iL3s;直接发育)。自由生活的雄性和雌性有性繁殖以产生被限制成为iL3的后代。在某些情况下,粪类杀伤性链球菌也可以进行自身感染,其中一些后寄生幼虫留在宿主肠道内,而不是通过粪便进入环境。这些幼虫可以在宿主体内发育成自身感染性幼虫(L3a),穿透肠壁,通过身体迁移,最终返回肠道成为生殖成虫。S. ratti的生命周期是相似的,除了S. ratti感染大鼠并且没有自身感染周期。环境生成是使用类圆线虫物种进行遗传研究的关键。自由生活的成年雌性(P0)可以显微注射;它们的后代,全部将成为iL3s,是潜在的F1转基因。这个数字是从Castelletto等人修改而来的。3. 请按此浏览此图的大图。
S. stercoralis 与其他胃肠道人寄生虫线虫共享其生物学的许多方面,包括宿主入侵和宿主免疫调节。例如, Necator 属和 Ancylostoma 属的人寄生虫钩虫也通过皮肤渗透感染,以类似的方式穿过身体,最终作为寄生成虫居住在小肠7中。因此,许多胃肠道线虫可能使用常见的感觉行为和免疫规避技术。因此,从 类圆线虫 中收集的知识将补充其他遗传上不太易处理的线虫的发现,并导致对这些复杂而重要的寄生虫有更完整的了解。
该显微注射方案概述了将DNA引入圆 线虫 自由生活成年雌性以制造转基因和突变后代的方法。描述了菌株维持要求,包括用于显微注射的成虫的发育时间和转基因后代的收集。包括实验方案和完整的显微注射技术演示,以及培养和筛选转基因后代的方案,以及所有必要设备和消耗品的清单。
Protocol
Representative Results
Discussion
该显微注射方案详细介绍了将转基因结构和CRISPR / Cas9介导的诱变引入 粪类链球菌 和 鼠李糖杆菌的方法。 对于粪 类葡萄球菌 和 鼠李松,注射后存活率和转基因或诱变率受制于几个可以微调的变量。
成功转基因的第一个关键考虑因素是如何构建质粒转基因。以前的研究发现,类 圆线虫 中外源转基因的表达需要使用 类圆线虫 5’启动子…
Disclosures
The authors have nothing to disclose.
Acknowledgements
pPV540和pPV402是宾夕法尼亚大学James Lok博士的善意礼物。我们感谢阿斯特拉·布莱恩特对手稿的有益评论。这项工作由Burroughs-Wellcome Fund Researchs in the Pathogenesis of Disease Award,Howard Hughes Medical Institute教师学者奖和美国国立卫生研究院R01 DC017959(E.A.H.)资助。
Materials
| (−)-Nicotine, ≥99% (GC), liquid | Sigma-Aldrich | N3876-5ML | nicotine for paralyzing worms |
| 3" iron C-clamp, 3" x 2" (capacity x depth) | VWR | 470121-790 | C-clamp to secure setup to bench top |
| Agarose LE | Phenix | RBA-500 | agarose for slides |
| Bone char, 4 lb pail, 10 x 28 mesh | Ebonex | n/a | charcoal for fecal-charcoal cultures |
| Bone char, granules, 10 x 28 mesh | Reade | bonechar10x28 | charcoal for fecal-cultures (alternative to the above) |
| Coarse micromanipulator | Narishige | MMN-1 | coarse micromanipulator |
| Corning Costar Spin-X centrifuge tube filters | Fisher | 07-200-385 | microfilter column |
| Cover glass, 48 x 60 mm, No. 1 thickness | Brain Research Lab | 4860-1 | coverslips (48 x 60 mm) |
| Deep Petri dishes, heavy version with 6 vents, 100 mm diameter | VWR | 82050-918 | 10 cm Petri dishes (for fecal-charcoal cultures) |
| Eisco retort base w/ rod | Fisher | 12-000-101 | stand for Baermann apparatus |
| Eppendorf FemtoJet microinjector microloader tips | VWR | 89009-310 | for filling microinjection needles |
| Fisherbrand absorbent underpads | Fisher | 14-206-62 | bench paper (for prepping) |
| Fisherbrand Cast-Iron Rings | Fisher | 14-050CQ | Baermann o-ring |
| Fisherbrand tri-cornered polypropylene beakers | Fisher | 14-955-111F | Plastic beaker (for mixing) |
| Fisherbrand tri-cornered polypropylene beakers | Fisher | 14-955-111F | Plastic beaker (for catch bucket/water bucket) |
| Fisherbrand tri-cornered polypropylene beakers | Fisher | 14-955-111F | Plastic beaker (x2) (to make holder) |
| Gorilla epoxie in syringe | McMaster-Carr | 7541A51 | glue (to attach tubing) |
| Halocarbon oil 700 | Sigma-Aldrich | H8898-50ML | halocarbon oil |
| High-temperature silicone rubber tubing for food and beverage, 1/2" ID, 5/8" OD | McMaster-Carr | 3038K24 | tubing (for funnel) |
| KIMAX funnels, long stem, 60° Angle, Kimble Chase | VWR | 89001-414 | Baermann funnel |
| Kimberly-Clark Professional Kimtech Science benchtop protectors | Fisher | 15-235-101 | bench paper (for prepping) |
| Leica stereomicroscope with fluorescence | Leica | M165 FC | GFP stereomicroscope for identifying and sorting transgenic worms |
| microINJECTOR brass straight arm needle-holder | Tritech | MINJ-4 | microinjection needle holder |
| microINJECTOR system | Tritech | MINJ-1 | microinjection system |
| Mongolian Gerbils | Charles River Laboratories | 213-Mongolian Gerbil | gerbils for maintenance of S. stercoralis, male 4-6 weeks |
| Nasco Whirl-Pak easy-to-close bags, 18 oz | VWR | 11216-776 | Whirl-Pak sample bags |
| Nylon tulle (mesh) | Jo-Ann Fabrics | zprd_14061949a | nylon mesh for Baermann holder |
| Platinum wire, 36 Gauge, per inch | Thomas Scientific | 1233S72 | platinum/iridium wire for worm picks |
| Puritan tongue depressors, 152 mm (L) x 17.5 mm (W) | VWR | 62505-007 | wood sticks (for mixing samples) |
| QIAprep Spin Miniprep Kit (250) | QIAGEN | 27106 | QIAGEN miniprep kit |
| Rats-Long Evans | Envigo | 140 HsdBlu:LE Long Evans | rats for maintenance of S. ratti, female 4-6 weeks |
| Rats-Sprague Dawley | Envigo | 002 Hsd:Sprague Dawley SD | rats for maintenance of S. ratti, female 4-6 weeks |
| Really Useful Boxes translucent storage boxes with lids, 1.6 L capacity, 7-5/8" x 5-5/16" x 4-5/16" | Office Depot | 452369 | plastic boxes for humidified chamber |
| Shepherd techboard, 8 x 16.5 inches | Newco | 999589 | techboard |
| Stainless steel raised wire floor | Ancare | R20SSRWF | wire cage bottoms |
| StalkMarket compostable cutlery spoons, 6", white, pack of 1,000 | Office Depot | 9587303 | spoons |
| Stender dish, stacking type, 37 x 25 mm | Carolina (Science) | 741012 | watch glasses (small, round) |
| Stereomicroscope | Motic | K-400 LED | dissecting prep scope |
| Storage tote, color clear/white, outside height 4-7/8 in, outside length 13-5/8 in, Sterilite | Grainger | 53GN16 | plastic boxes for humidified chamber |
| Sutter P-30 micropipette puller | Sutter | P-30/P | needle puller with platinum/iridium filament |
| Syracuse watch glasses | Fisher | S34826 | watch glasses (large, round) |
| Thermo Scientific Castaloy fixed-angle clamps | Fisher | 05-769-2Q | funnel clamps (2x) |
| Three-axis hanging joystick oil hydrolic micromanipulator | Narishige | MM0-4 | fine micromanipulator |
| United Mohr pinchcock clamps | Fisher | S99422 | Pinch clamps (2x) |
| Vented, sharp-edge Petri dishes (60 mm diameter) | Tritech Research | T3308P | 6 cm Petri dishes (for small-scale fecal-charcoal cultures) |
| VWR light-duty tissue wipers | VWR | 82003-820 | lining for Baermann holder |
| watch glass, square, 1-5/8 in | Carolina (Science) | 742300 | watch glasses (small, square) |
| Whatman qualitative grade plain circles, grade 1, 5.5 cm diameter | Fisher | 09-805B | filter paper (for 6 cm Petri dishes) |
| Whatman qualitative grade plain circles, grade 1, 9 cm diameter | Fisher | 09-805D | filter paper (for 10 cm Petri dishes) |
| World Precision Instrument borosilicate glass capillary, 1.2 mm x 4 in | Fisher | 50-821-813 | glass capillaries for microinjection needles |
| X-Acto Knives, No. 1 Knife With No. 11 Blade | Office Depot | 238816 | X-Acto knives without blades to hold worm picks |
| Zeiss AxioObserver A1 | Zeiss | n/a | inverted microscope |
References
- Krolewiecki, A. J., et al. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full. PLoS Neglected Tropical Diseases. 7 (5), 2165 (2013).
- Buonfrate, D., et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 9 (6), 468 (2020).
- Castelletto, M. L., Gang, S. S., Hallem, E. A. Recent advances in functional genomics for parasitic nematodes of mammals. Journal of Experimental Biology. 223, 206482 (2020).
- Evans, T. C., et al. Transformation and microinjection. WormBook. , (2006).
- Lok, J. B., Unnasch, T. R., et al. Transgenesis in animal parasitic nematodes: Strongyloides spp. and Brugia spp. WormBook. , (2013).
- Shao, H. G., Li, X. S., Lok, J. B. Heritable genetic transformation of Strongyloides stercoralis by microinjection of plasmid DNA constructs into the male germline. International Journal for Parasitology. 47 (9), 511-515 (2017).
- Schafer, T. W., Skopic, A. Parasites of the small intestine. Current Gastroenterology Reports. 8 (4), 312-320 (2006).
- Stiernagle, T. Maintenance of C. elegans. The C. elegans Research Community, WormBook. , (2006).
- Gang, S. S., et al. Targeted mutagenesis in a human-parasitic nematode. PLoS Pathogens. 13 (10), 1006675 (2017).
- Lok, J. B. Strongyloides stercoralis: a model for translational research on parasitic nematode biology. The C. elegans Research Community, WormBook. , (2007).
- Hawdon, J. M., Schad, G. A. Long-term storage of hookworm infective larvae in buffered saline solution maintains larval responsiveness to host signals. Proceedings of the Helminthological Society of Washington (USA). 58 (1), 140-142 (1991).
- Bargmann, C. I., Hartwieg, E., Horvitz, H. R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 74 (3), 515-527 (1993).
- Junio, A. B., et al. Strongyloides stercoralis: cell- and tissue-specific transgene expression and co-transformation with vector constructs incorporating a common multifunctional 3′ UTR. Experimental Parasitology. 118 (2), 253-265 (2008).
- Gang, S. S., et al. Chemosensory mechanisms of host seeking and infectivity in skin-penetrating nematodes. Proceedings of the National Academy of Sciences of the United States of America. 117 (30), 17913-17923 (2020).
- Bryant, A. S., et al. A critical role for thermosensation in host seeking by skin-penetrating nematodes. Current Biology. 28 (14), 2338-2347 (2018).
- Lok, J. B. Nucleic acid transfection and transgenesis in parasitic nematodes. Parasitology. 139 (5), 574-588 (2012).
- Shao, H., et al. Transposon-mediated chromosomal integration of transgenes in the parasitic nematode Strongyloides ratti and establishment of stable transgenic lines. PLoS Pathogens. 8 (8), 1002871 (2012).
- Lok, J. piggyBac: a vehicle for integrative DNA transformation of parasitic nematodes. Mobile Genetic Elements. 3 (2), 24417 (2013).
- Li, X., et al. Successful transgenesis of the parasitic nematode Strongyloides stercoralis requires endogenous non-coding control elements. International Journal for Parasitology. 36 (6), 671-679 (2006).
- Bryant, A. S., Hallem, E. A. The Wild Worm Codon Adapter: a web tool for automated codon adaptation of transgenes for expression in non-Caenorhabditis nematodes. G3. 3 (7), (2021).
- Crane, M., et al. In vivo measurements reveal a single 5′-intron is sufficient to increase protein expression level in Caenorhabditis elegans. Scientific Reports. 9 (1), 9192 (2019).
- Han, Z., et al. Improving transgenesis efficiency and CRISPR-associated tools through codon optimization and native intron addition in Pristionchus nematodes. Genetics. 216 (4), 947-956 (2020).
- Adams, S., Pathak, P., Shao, H., Lok, J. B., Pires-daSilva, A. Liposome-based transfection enhances RNAi and CRISPR-mediated mutagenesis in non-model nematode systems. Scientific Reports. 9 (1), 483 (2019).
- Dulovic, A., Puller, V., Streit, A. Optimizing culture conditions for free-living stages of the nematode parasite Strongyloides ratti. Experimental Parasitology. 168, 25-30 (2016).
- Harvey, S. C., Gemmill, A. W., Read, A. F., Viney, M. E. The control of morph development in the parasitic nematode Strongyloides ratti. Proceedings of the Royal Society B: Biological Sciences. 267 (1457), 2057-2063 (2000).
- Kim, A., Pyykko, I. Size matters: versatile use of PiggyBac transposons as a genetic manipulation tool. Molecular and Cellular Biochemistry. 354 (1-2), 301-309 (2011).
- Lok, J. B., Shao, H., Massey, H. C., Li, X. Transgenesis in Strongyloides and related parasitic nematodes: historical perspectives, current functional genomic applications and progress towards gene disruption and editing. Parasitology. 144 (3), 327-342 (2017).
- Farboud, B., Meyer, B. J. Dramatic enhancement of genome editing by CRISPR/Cas9 through improved guide RNA design. Genetics. 199 (4), 959-971 (2015).
- Cheong, M. C., et al. Identification of a nuclear receptor/coactivator developmental signaling pathway in the nematode parasite Strongyloides stercoralis. Proceedings of the National Academy of Sciences of the United States of America. 118 (8), 2021864118 (2021).
- Nolan, T. J., Megyeri, Z., Bhopale, V. M., Schad, G. A. Strongyloides stercoralis: the first rodent model for uncomplicated and hyperinfective strongyloidiasis, the Mongolian gerbil (Meriones unguiculatus). Journal of Infectious Diseases. 168 (6), 1479-1484 (1993).
- Li, X., et al. Transgenesis in the parasitic nematode Strongyloides ratti. Molecular and Biochemical Parasitology. 179 (2), 114-119 (2011).
- Viney, M. E. Exploiting the life cycle of Strongyloides ratti. Parasitology Today. 15 (6), 231-235 (1999).
- Stoltzfus, J. D., Massey, H. C., Nolan, T. J., Griffith, S. D., Lok, J. B. Strongyloides stercoralis age-1: a potential regulator of infective larval development in a parasitic nematode. PLoS ONE. 7 (6), 38587 (2012).
- Castelletto, M. L., Massey, H. C., Lok, J. B. Morphogenesis of Strongyloides stercoralis infective larvae requires the DAF-16 ortholog FKTF-1. PLoS Pathogens. 5 (4), 1000370 (2009).
- Douglas, B., et al. Transgenic expression of a T cell epitope in Strongyloides ratti reveals that helminth-specific CD4+ T cells constitute both Th2 and Treg populations. PLoS Pathogens. 17 (7), 1009709 (2021).

