发育(青蛙)胚胎中的细胞谱系引导质谱蛋白质组学
Summary
在这里,我们描述了脊椎动物非洲 爪蟾 胚胎中具有已知组织命运的细胞谱系的基于质谱的蛋白质组学表征。
Abstract
当细胞产生组织和器官时,分子事件的表征提高了更好地了解正常发育和设计有效的疾病疗法的潜力。能够准确鉴定和定量各种类型和大量蛋白质的技术将提供关于在空间和时间上协调组织和生物体发育的分子机制的信息。在这里,我们提出了一种基于质谱的方案,该协议能够测量非洲 爪 蟾(青蛙)胚胎中已鉴定细胞谱系中的数千种蛋白质。该方法建立在可重复的细胞命运图谱和既定方法的基础上,以从这种脊椎动物发育模型中鉴定,荧光标记,跟踪和采样细胞及其后代(克隆)。使用微量采样或通过解剖或荧光激活细胞分选分离细胞收集细胞内容物后,提取并处理蛋白质以进行自下而上的蛋白质组学分析。液相色谱和毛细管电泳用于通过高分辨率质谱(HRMS)为蛋白质检测和定量提供可扩展的分离。为神经组织命运细胞的蛋白质组学表征提供了代表性的例子。细胞谱系引导的HRMS蛋白质组学适用于不同的组织和生物体。它具有足够的灵敏度、特异性和定量性,可以窥探脊椎动物发育过程中蛋白质组的时空动态。
Introduction
我们对细胞分化以及组织和器官起源的理解是数十年来精心设计的基因及其产物靶向筛选的结果。在重要的细胞事件中,增加我们对所有生物分子及其数量的了解将有助于解开控制脊椎动物身体计划的空间和时间模式的分子机制。能够进行分子扩增和测序的技术现在能够定期报告大量基因和转录本,支持基础生物学和转化研究中的假设驱动研究。为了理解发展中的系统,转录和翻译之间的复杂关系主张直接分析多种蛋白质及其翻译后修饰。使用 体外 生物系统(如诱导多能干细胞)的全球蛋白质组学开始描绘组织诱导的机制1,2。在复杂的生物中,例如脊椎动物胚胎,发育依赖于空间和时间背景下的形态原梯度3。因此,随着细胞分化形成专门的组织(如神经组织),获得蛋白质组学变化的知识,为解锁控制正常和缺陷发育的分子程序和指导下一代疗法提供了一把钥匙。
脊椎动物南非爪蛙(非洲爪蟾)是细胞和发育、神经和再生生物学的成熟模型。约翰·戈登爵士(Sir John Gurdon)因发现体细胞核的多能性而获得2012年诺贝尔生理学或医学奖4,5,强调了该模型对基础和转化研究发现的重要性。非洲爪蟾胚胎在母体外部发育,从而促进细胞、细胞克隆和基因表达在不同发育阶段的直接操作。不对称的色素沉着和刻板的细胞分裂使得能够绘制出来自 16-6 和 32 细胞7,8 期胚胎的可重复命运图。对于基于高分辨率质谱(HRMS)的蛋白质组学,该模型的其他优点包括相对较大的尺寸(直径~1毫米),这会产生丰富的蛋白质含量进行分析(早期切割阶段胚胎~130μg,16细胞胚胎的单细胞中~10μg蛋白质含量)9,10。
目前,HRMS是检测蛋白质的首选领先技术。该技术能够直接、灵敏、特异性地检测和定量多种(通常是数百到数千种不同的蛋白质)11。HRMS自下而上的蛋白质组学涉及一系列相互关联的步骤。从细胞/组织样品中提取后,用蛋白水解酶(如胰蛋白酶(自下而上的蛋白质组学))消化蛋白质。所得肽根据其不同的物理化学性质进行分离,包括疏水性(反相液相色谱,LC),净电荷(离子交换色谱),大小(尺寸排阻色谱)或电泳迁移率(毛细管电泳,CE)。然后对肽进行充电(电离),通常使用电喷雾电离(ESI),并通过串联HRMS通过气相碎裂检测和测序肽离子。所得肽数据映射到所研究生物体的蛋白质组。由于蛋白质特异性(蛋白型)肽离子信号强度与浓度相关,蛋白质定量可以无标记或基于标记(多重定量)进行。HRMS蛋白质组学产生了有关所研究系统分子状态的丰富信息资源,允许生成假设和后续功能研究。
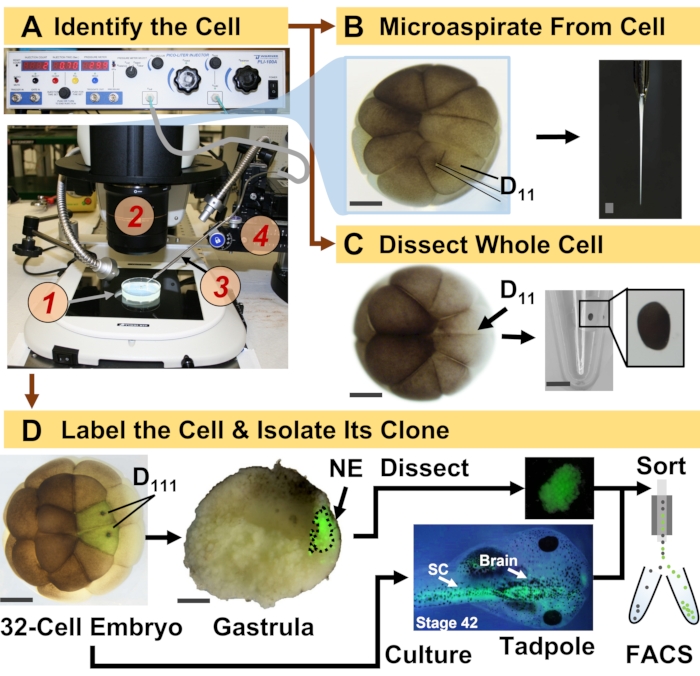
图 1:时空可扩展的蛋白质组学,使细胞谱系引导发育(青蛙)胚胎中的 HRMS 蛋白质组学成为可能。 (A)在平移阶段(4)的控制下,使用体视显微镜(2)注射已识别的细胞(插图)的样品(1)用于注射已识别的细胞(插图)。(B)对16细胞胚胎中鉴定的左D11细胞进行亚细胞采样。(C)从16细胞胚胎中解剖整个D11细胞。(D)从32细胞胚胎中对左右D111后代进行荧光(绿色)示踪,以指导解剖原肠胚中的神经外胚层(NE)(第10阶段),并使用FACS从蝌蚪中分离后代组织。比例尺:胚胎200μm,小瓶1.25mm。数字经参考文献15,19,21,59许可改编。请点击此处查看此图的大图。
这里介绍的方案能够基于HRMS对发育中的X. laevis胚胎中鉴定的细胞/组织中的大量蛋白质进行定量。该方法建立在准确的细胞鉴定、可重复的细胞命运图谱和在该生物模型中跟踪细胞谱系的既定方法之上6,7,8。如图1所示,我们通过采用全细胞解剖或毛细管微量采样来抽吸细胞内容物来研究来自单细胞的蛋白质组。监测细胞的谱系使我们能够研究蛋白质组的时空演变,因为细胞在原肠胚形成过程中形成组织。通过注射与惰性葡聚糖或mRNA偶联的荧光团来标记细胞后代,以用于荧光蛋白(例如,绿色荧光蛋白或GFP)。标记的后代在所需的发育时间点分离。在原肠胚形成过程中,可以通过解剖分离紧密聚集的细胞克隆。原肠胚形成后,由于迁移运动,细胞克隆可能分布在胚胎内,并且可以通过荧光激活细胞分选(FACS)从解离组织中分离出来。这些细胞和组织中的蛋白质通过自下而上的蛋白质组学进行测量,采用HPLC或CE进行分离,ESI串联HRMS进行鉴定。细胞谱系引导的HRMS蛋白质组学可扩展到胚胎内的不同细胞大小和谱系,并且具有特异性、灵敏度和定量性。通过此处显示的精选示例,我们还证明了该协议具有可扩展性,并且广泛适用于不同类型的细胞和细胞谱系。
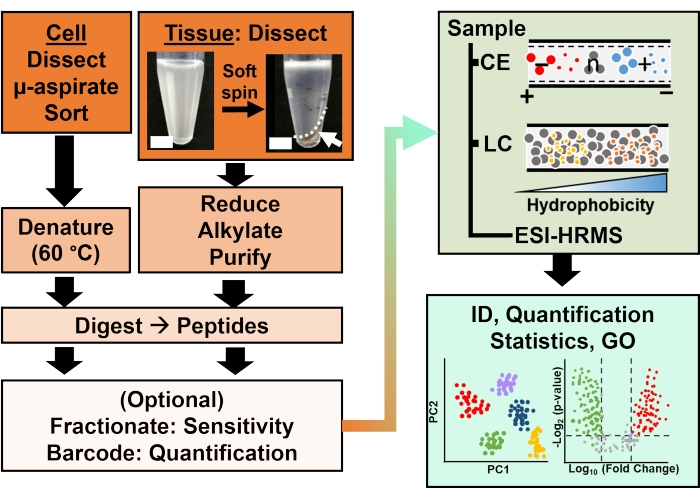
图 2:生物分析工作流程。 显微解剖和毛细管抽吸,或FACS有助于细胞和克隆蛋白含量的采样。使用电喷雾电离 (ESI) 高分辨率质谱 (HRMS) 去除丰度蛋黄蛋白并通过毛细管电泳 (CE) 或纳流液相色谱 (LC) 增强鉴定 (ID) 灵敏度进行分离。量化揭示了失调,结合基因本体(GO)提供的信息,为假设驱动的研究提供了新的信息。数字经参考文献15许可改编。 请点击此处查看此图的大图。
Protocol
Representative Results
Discussion
该协议能够表征 非洲爪蟾 物种胚胎中已鉴定细胞谱系中的蛋白质表达。该方法源于HRMS,结合了分子鉴定的精湛特异性,无需分子探针即可进行多蛋白检测的能力(通常是数百到数千种不同的蛋白质)以及定量能力。对细胞和发育(神经)生物学中经典工具和工作流程的适应性将HRMS蛋白质组学扩展到令人兴奋的应用,包括脊椎动物X . laevis 胚胎中干细胞分化的整体表征。
<p class="j…Disclosures
The authors have nothing to disclose.
Acknowledgements
我们感谢Jie Li(马里兰大学帕克分校)关于胚胎解离和FACS的宝贵讨论。我们感谢Vi M. Quach和Camille Lombard-Banek在先前的研究中为样品制备和数据收集提供的帮助,这些研究举例说明了本协议中强调的蛋白质组学应用。这项工作的部分内容得到了美国国家科学基金会的支持,奖励号为IOS-1832968 CAREER(授予P.N.),美国国立卫生研究院的奖励号为R35GM124755(授予P.N.),马里兰大学 – 国家癌症研究所合作计划(授予P.N.)和COSMOS俱乐部基金会研究奖(授予ABB和L.R.P.)。
Materials
| Acetonitrile (LC-MS-grade) | Fisher Scientific | A955 | |
| Agarose | ThermoFisher Scientific | R0492 | |
| Ammonium bicarbonate | Fisher Scientific | A643-500 | |
| Analytical Column | Thermo Scientific | 164941 | |
| Analytical microbalance | Mettler-Toledo | XSE105DU | |
| Automatic peptide fractionation platform | Agilent | 1260 Infinity II | |
| Borosilicate Capillaries | Sutter Instruments Co. | B100-50-10 | |
| Borosilicate Capillaries (for making Emmitters) | Sutter Instruments | B100-75-10 | |
| C18 spin columns (for desalting) | ThermoFisher Scientific | 89870 | |
| Camera ro monitor electrospray | Edmund Optics Inc. | EO-2018C | |
| Combretastatin A4 | Millipore Sigma | C7744 | |
| Commercial CESI system | AB SCIEX | CESI | |
| (Cyclohexylamino)-1-propanesulfonic acid (CAPS) | VWR | 97061-492 | |
| Cytochalasin D | Millipore Sigma | C8273 | |
| Dextran, Alexa Fluor 488; 10,000 MW, Anionic, Fixable | ThermoFisher Scientific | D22910 | |
| Diothiothreitol | Fisher Scientific | FERR0861 | |
| Dumont #5 Forceps | Fine Science Tools | 11252-30 | |
| EDTA | Fisher Scientific | AAJ62786AP | |
| Epifluorescence light source | Lumencore | AURA III | |
| Eppendorf LoBing microcentrifuge tubes: protein | Fisher Scientific | 13-698-793 | |
| Formic acid (LC-MS-grade) | Fisher Scientific | A117-50 | |
| Freezer (-20 °C) | Fisher Scientific | 97-926-1 | |
| Freezer (-80 °C) | Thermo Scientific | TSX40086A | |
| Fused silica capillary | Molex | 1088150596 | |
| Heat Block | Benchmark | BSH300 | |
| High pressure liquid Chromatography System | ThermoFisher Scientific | Dionex Ultimate 3000 RSLC nanosystem | |
| High voltage power supply | Spellman | CZE1000R | |
| High-resolution Mass Spectrometer | ThermoFisher Scientific | Orbitrap Fusion Lumos Tribrid Mass Spectrometer | |
| HPLC caps | Thermo Scientific | C4013-40A | |
| HPLC Vials | Thermo Scientific | C4013-11 | |
| Illuminator e.g. Goosenecks | Nikon | C-FLED2 | |
| Ingenuity Pathway Analysis | Qiagen | ||
| Iodoacetamide | Fisher Scientific | AC122275000 | |
| Methanol (LC-MS-grade) | Fisher Scientific | A456 | |
| Methanol (LC-MS-grade) | Fisher Scientific | A456-4 | |
| Microcapillary puller | Suttor Instruments | P-2000 | |
| Microinjector | Warner Instrument, Handem, CT | PLI-100A | |
| Micropippette puller | Sutter Instruments Co. | P-1000 | |
| MS data analysis software, commercial | ProteomeDiscoverer | ||
| MS data analysis software, opensource | MaxQuant | ||
| non-idet 40 substitute | Millipore Sigma | 11754599001 | |
| Petri dish 60 mm and 80 mm | Fisher Scientific | S08184 | |
| Pierce 10 µL bed Zip-tips (for desalting) | ThermoFisher Scientific | 87782 | |
| Pierce bicinchoninic acid protein assay kit | ThermoFisher Scientific | 23225 | |
| Pierce quantitative colorimetric peptide assay | ThermoFisher Scientific | 23275 | |
| Pierce Trypsin Protease (MS Grade) | Fisher Scientific | PI90058 | |
| Protein LoBind vials | Eppendorf | 0030108434 , 0030108442 |
|
| Refrigerated Centrifuge | Eppendorf | 5430R | |
| Refrigerated Incubator | Thermo Scientific | PR505755R/3721 | |
| sodium isethionate | Millipore Sigma | 220078 | |
| sodium pyrophosphate | Sigma Aldrich | 221368-100G | |
| Stainless steel BGE vial | Custom-Built | ||
| Stainless steel sample vials | Custom-Built | ||
| Stereomicroscope (objective 10x) | Nikon | SMZ 1270, SZX18 | |
| Sucrose | VWR | 97063-790 | |
| Syringe pumps (2) | Harvard Apparatus | 704506 | |
| Syringes (gas-tight): 500–1000 µL | Hamilton | 1750TTL | |
| Transfer pipettes (Plastic, disposable) | Fisher Scientific | 13-711-7M | |
| Trap Column | Thermo Scientific | 164750 | |
| Tris-HCl (1 M solution) | Fisher Scientific | AAJ22638AP | |
| Vacuum concentrator capable of operation at 4–10 °C | Labconco | 7310022 | |
| Vortex-mixer | Benchmark | BS-VM-1000 | |
| Water (LC-MS-grade) | Fisher Scientific | W6 | |
| Water (LC-MS-grade) | Fisher Scientific | W6 | |
| XYZ translation stage | Thorlabs | PT3 | |
| XYZ translation stage | Custom-Built |
References
- Shoemaker, L. D., Kornblum, H. I. Neural Stem Cells (NSCs) and Proteomics. Molecular & Cellular Proteomics. 15 (2), 344-354 (2016).
- Cervenka, J., et al. Proteomic characterization of human neural stem cells and their secretome during in vitro differentiation. Frontiers in Cellular Neuroscience. 14, 612560 (2021).
- Christian, J. L. Morphogen gradients in development: From form to function. Wiley Interdisciplinary Reviews. Developmental Biology. 1 (1), 3-15 (2012).
- Gurdon, J. B., Elsdale, T. R., M, F. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 182, 64-65 (1958).
- Harland, R. M., Grainger, R. M. Xenopus research: metamorphosed by genetics and genomics. Trends in Genetics. 27 (12), 507-515 (2011).
- Moody, S. A. Fates of the blastomeres of the 16-cell stage Xenopus embryo. 发育生物学. 119 (2), 560-578 (1987).
- Moody, S. A. Fates of the blastomeres of the 32-cell stage Xenopus embryo. 发育生物学. 122 (2), 300-319 (1987).
- Dale, L., Slack, J. M. W. Fate map for the 32-cell stage of Xenopus laevis. Development. 99 (4), 527-551 (1987).
- Sun, L. L., et al. Single cell proteomics using frog (Xenopus laevis) blastomeres isolated from early stage embryos, which form a geometric progression in protein content. Analytical Chemistry. 88 (13), 6653-6657 (2016).
- Lombard-Banek, C., Moody, S. A., Nemes, P. Single-cell mass spectrometry for discovery proteomics: quantifying translational cell heterogeneity in the 16-cell frog (Xenopus) embryo. Angewandte Chemie-International Edition. 55 (7), 2454-2458 (2016).
- Zhang, Y. Y., Fonslow, B. R., Shan, B., Baek, M. C., Yates, J. R. Protein analysis by shotgun/bottom-up proteomics. Chemical Reviews. 113 (4), 2343-2394 (2013).
- Sive, H. L., Grainger, R. M., Harland, R. M. . Early development of Xenopus laevis: A laboratory manual. , (2000).
- Briggs, J. A., et al. The dynamics of gene expression in vertebrate embryogenesis at single-cell resolution. Science. 360 (6392), (2018).
- Gupta, M., Sonnett, M., Ryazanova, L., Presler, M., Wuhr, M., Vleminckx, K. Quantitative proteomics of xenopus embryos I, sample preparation. Xenopus. Methods in Molecular Biology. 1865, 175-194 (2018).
- Baxi, A. B., Lombard-Banek, C., Moody, S. A., Nemes, P. Proteomic characterization of the neural ectoderm fated cell clones in the Xenopus laevis embryo by high-resolution mass spectrometry. ACS Chemical Neuroscience. 9 (8), 2064-2073 (2018).
- Moody, S. A. Cell lineage analysis in Xenopus embryos. Methods in Molecular Biology. 135, 331-347 (2000).
- Sater, A. K., Moody, S. A. Using Xenopus to understand human diseases and developmental disorders. Genesis. 55 (1-2), 1-14 (2017).
- Lombard-Banek, C., Choi, S. B., Nemes, P., Allbritton, N. L., Kovarik, M. L. . Enzyme Activity in Single Cells. Methods in Enzymology. 628, 263-292 (2019).
- Lombard-Banek, C., Moody, S. A., Nemes, P. High-sensitivity mass spectrometry for probing gene translation in single embryonic cells in the early frog (Xenopus) embryo. Frontiers in Cell and Developmental Biology. 4, 11 (2016).
- Onjiko, R. M., Portero, E. P., Moody, S. A., Nemes, P. Microprobe capillary electrophoresis mass spectrometry for single-cell metabolomics in live frog (Xenopus laevis) embryos. Journal of Visualized Experiments: JoVE. (130), e56956 (2017).
- Lombard-Banek, C., Moody, S. A., Manzin, M. C., Nemes, P. Microsampling capillary electrophoresis mass spectrometry enables single-cell proteomics in complex tissues: developing cell clones in live Xenopus laevis and zebrafish embryos. Analytical Chemistry. 91 (7), 4797-4805 (2019).
- Klein, S. L. The first cleavage furrow demarcates the dorsal-ventral axis in Xenopus embryos. 发育生物学. 120 (1), 299-304 (1987).
- Karimi, K., et al. Xenbase: a genomic, epigenomic and transcriptomic model organism database. Nucleic Acids Research. 46 (1), 861-868 (2018).
- Kakebeen, A. D., Chitsazan, A. D., Wills, A. E. Tissue disaggregation and isolation of specific cell types from transgenic Xenopus appendages for transcriptional analysis by FACS. Developmental Dynamics. 250 (9), 1381-1392 (2021).
- Garcia, B. A. What does the future hold for top down mass spectrometry. Journal of the American Society for Mass Spectrometry. 21 (2), 193-202 (2010).
- Toby, T. K., Fornelli, L., Kelleher, N. L. Progress in top-down proteomics and the analysis of proteoforms. Annual Review of Analytical Chemistry. (Palo Alto Calif). 9 (1), 499-519 (2016).
- Zhang, Z. B., Dubiak, K. M., Huber, P. W., Dovichi, N. J. Miniaturized filter-aided sample preparation (MICRO-FASP) method for high throughput, ultrasensitive proteomics sample preparation reveals proteome asymmetry in Xenopus laevis Embryos. Analytical Chemistry. 92 (7), 5554-5560 (2020).
- Wisniewski, J. R., Becher, D. . Microbial Proteomics: Methods and Protocols.Methods in Molecular Biology. 1841, 3-10 (2018).
- Hughes, C. S., et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nature Protocols. 14 (1), 68-85 (2019).
- Zhu, Y., et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10-100 mammalian cells. Nature Communications. 9, 882 (2018).
- Wessel, D., Flugge, U. I. A method for the quantitative recovery of protein in dilute-solution in the presence of detergents and lipids. Analytical Biochemistry. 138 (1), 141-143 (1984).
- Jiang, L., He, L., Fountoulakis, M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. Journal of Chromatography A. 1023 (2), 317-320 (2004).
- Hildonen, S., Halvorsen, T. G., Reubsaet, L. Why less is more when generating tryptic peptides in bottom-up proteomics. Proteomics. 14 (17-18), 2031-2041 (2014).
- Budnik, B., Levy, E., Harmange, G., Slavov, N. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biology. 19, 161 (2018).
- Drouin, N., et al. Capillary electrophoresis-mass spectrometry at trial by metabo-ring: effective electrophoretic mobility for reproducible and robust compound annotation. Analytical Chemistry. 92 (20), 14103-14112 (2020).
- Sun, L. L., Zhu, G. J., Zhang, Z. B., Mou, S., Dovichi, N. J. Third-generation electrokinetically pumped sheath-flow nanospray interface with improved stability and sensitivity for automated capillary zone electrophoresis-mass spectrometry analysis of complex proteome digests. Journal of Proteome Research. 14 (5), 2312-2321 (2015).
- DeLaney, K., Sauer, C. S., Vu, N. Q., Li, L. J. Recent advances and new perspectives in capillary electrophoresis-mass spectrometry for single cell "omics". Molecules. 24 (1), 21 (2019).
- Nemes, P., Rubakhin, S. S., Aerts, J. T., Sweedler, J. V. Qualitative and quantitative metabolomic investigation of single neurons by capillary electrophoresis electrospray ionization mass spectrometry. Nature Protocols. 8 (4), 783-799 (2013).
- Choi, S. B., Zamarbide, M., Manzini, M. C., Nemes, P. Tapered-tip capillary electrophoresis nano-electrospray ionization mass spectrometry for ultrasensitive proteomics: the mouse cortex. Journal of the American Society for Mass Spectrometry. 28 (4), 597-607 (2017).
- Pino, L. K., Rose, J., O’Broin, A., Shah, S., Schilling, B. Emerging mass spectrometry-based proteomics methodologies for novel biomedical applications. Biochemical Society Transactions. 48 (5), 1953-1966 (2020).
- Chen, C., Hou, J., Tanner, J. J., Cheng, J. L. Bioinformatics methods for mass spectrometry-based proteomics data analysis. International Journal of Molecular Sciences. 21 (8), 25 (2020).
- Peshkin, L., et al. On the relationship of protein and mRNA dynamics in vertebrate embryonic development. Developmental Cell. 35 (3), 383-394 (2015).
- Cox, J., et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Molecular & Cellular Proteomics. 13 (9), 2513-2526 (2014).
- Gygi, S. P., et al. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nature Biotechnology. 17 (10), 994-999 (1999).
- Thompson, A., et al. Tandem mass tags: A novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Analytical Chemistry. 75 (8), 1895-1904 (2003).
- Mi, H. Y., et al. PANTHER version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive api. Nucleic Acids Research. 49, 394-403 (2021).
- Schmidt, E., et al. . On the Move Federated Workshops. , 710-719 (2006).
- Deutsch, E. W., et al. Trans-Proteomic pipeline, a standardized data processing pipeline for large-scale reproducible proteomics informatics. Proteomics Clinical Applications. 9 (7-8), 745-754 (2015).
- Tyanova, S., et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nature Methods. 13 (9), 731-740 (2016).
- Demsar, J., et al. Orange: Data mining toolbox in Python. Journal of Machine Learning Research. 14, 2349-2353 (2013).
- Oberg, A. L., Vitek, O. Statistical design of quantitative mass spectrometry-based proteomic experiments. Journal of Proteome Research. 8 (5), 2144-2156 (2009).
- Jensen, L. J., et al. STRING 8 – a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Research. 37, 412-416 (2009).
- Schweppe, D. K., Huttlin, E. L., Harper, J. W., Gygi, S. P. BioPlex display: an interactive suite for large-scale AP-MS protein-protein interaction data. Journal of Proteome Research. 17 (1), 722-726 (2018).
- Hornbeck, P. V., et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Research. 43, 512-520 (2015).
- Letunic, I., Khedkar, S., Bork, P. SMART: recent updates, new developments and status in 2020. Nucleic Acids Research. 49, 458-460 (2021).
- Lombard-Banek, C., et al. In vivo subcellular mass spectrometry enables proteo-metabolomic single-cell systems biology in a chordate embryo developing to a normally behaving tadpole (X. laevis). Angewandte Chemie-International Edition. 60 (23), 12852-12858 (2021).
- Lombard-Banek, C., Reddy, S., Moody, S. A., Nemes, P. Label-free quantification of proteins in single embryonic cells with neural fate in the cleavage-stage frog (Xenopus laevis) embryo using capillary electrophoresis electrospray ionization high-resolution mass spectrometry (CE-ESI-HRMS). Molecular & Cellular Proteomics. 15 (8), 2756-2768 (2016).
- Saha-Shah, A., et al. Single cell proteomics by data-independent acquisition to study embryonic asymmetry in Xenopus laevis. Analytical Chemistry. 91 (14), 8891-8899 (2019).
- Onjiko, R. M., Portero, E. P., Moody, S. A., Nemes, P. In situ microprobe single-cell capillary electrophoresis mass spectrometry: metabolic reorganization in single differentiating cells in the live vertebrate (Xenopus laevis) embryo. Analytical Chemistry. 89 (13), 7069-7076 (2017).
- Perez-Riverol, Y., et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Research. 47, 442-450 (2019).

