Stimulation of Stem Cell Niches and Tissue Regeneration in Mouse Skin by Switchable Protoporphyrin IX-Dependent Photogeneration of Reactive Oxygen Species In Situ
Summary
The aim of this protocol is to induce transient in vivo production of nonlethal levels of reactive oxygen species (ROS) in mouse skin, further promoting physiological responses in the tissue.
Abstract
Here, we describe a protocol to induce switchable in vivo photogeneration of endogenous reactive oxygen species (ROS) in mouse skin. This transient production of ROS in situ efficiently activates cell proliferation in stem cell niches and stimulates tissue regeneration as strongly manifested through the acceleration of burn healing and hair follicle growth processes. The protocol is based on a regulatable photodynamic treatment that treats the tissue with precursors of the endogenous photosensitizer protoporphyrin IX and further irradiates the tissue with red light under tightly controlled physicochemical parameters. Overall, this protocol constitutes an interesting experimental tool to analyze ROS biology.
Introduction
Reactive oxygen species (ROS) are the result of the chemical reduction of molecular oxygen to form water, and include singlet oxygen, superoxide anion, hydrogen peroxide and the hydroxyl radical1,2,3. ROS have a very short lifespan due to their extremely chemical reactive nature. In aerobic organisms, ROS are incidentally formed inside the cells as a major leaky by-product of aerobic respiration (electron transport chain) in the mitochondria. Transient accumulation of high levels of ROS in the cell results in an oxidative stress condition that may provoke the irreversible inactivation of proteins, lipids and sugars and the introduction of mutations in the DNA molecule2,3,4,5. The gradual accumulation of oxidative damage in cells, tissues and whole organisms steadily increases with time and has been associated with the induction of cell death programs, several pathologies, and the ageing process2,3,4,6.
Aerobic organisms have steadily evolved efficient molecular mechanisms to tackle excess ROS accumulation in cells and tissues. These mechanisms include members of the superoxide dismutase (SOD) protein family, which catalyze superoxide radical dismutation into molecular oxygen and hydrogen peroxide, as well as different catalases and peroxidases which use the antioxidant pool (glutathione, NADPH, peroxiredoxin, thioredoxin7,8) to catalyze the subsequent conversion of hydrogen peroxide to water and molecular oxygen.
However, several reports support the role of ROS as key components of molecular circuits that regulate critical cell functions, including proliferation, differentiation and mobility2,3,4. This concept is further supported by the initial identification and characterization of dedicated ROS-producing mechanisms in aerobic organisms, including lipoxygenases cyclooxygenases and NADPH oxidases9,10. In this sense, ROS exhibit an active role during vertebrate embryo development11,12,13 and key roles for these molecules in the regulation of specific in vivo physiological functions have been reported in different experimental systems, including the differentiation program of hematopoietic progenitors in Drosophila14, healing induction in zebrafish, or tail regeneration in Xenopus tadpoles15. In mammals, ROS have been involved in the self-renewal/differentiation potential of neural stem cells in a neurosphere model16 and in the deregulation of intestinal stem cell function during colorectal cancer initiation17. In the skin, ROS signalling has been associated with epidermal differentiation and the regulation of the skin stem cell niche and the hair follicle growth cycle18,19.
In this perspective, a major experimental limitation to determine the physiological roles of ROS in biological systems, both in normal or pathological conditions, is the lack of adequate experimental tools to induce controlled production of these molecules in cells and tissues, accurately resembling their physiological production as second signalling messengers. At present, most experimental approaches involve the administration of exogenous ROS, mostly in the form of hydrogen peroxide. We have recently implemented an experimental approach to switch on a transient, nonlethal in vivo production of endogenous ROS in the mouse skin, based on the administration of precursors of the endogenous photosensitizer protoporphyrin IX (PpIX; e.g., aminolaevulinic acid or its methyl derivative methylaminolevulinate) and further irradiation of the sample with red light to induce the in situ formation of ROS from intracellular molecular oxygen (Figure 1). This photodynamic procedure may be efficiently used to stimulate resident stem cell niches, thus activating the regenerative programs of the tissue19,20 and opening the way for new therapeutic modalities in skin regenerative medicine. Here, we present a detailed description of the protocol, showing representative examples of stimulation of stem cell niches, measured as an increase in the number of long-term 5-bromo-2’-deoxyuridine (BrdU) label retaining cells (LRCs) in the bulge region of the hair follicle19,21, and subsequent activation of regeneration programs (acceleration of hair growth and burn healing processes) induced by transient, nonlethal ROS production in the skin of C57Bl6 mouse strain.
Protocol
All mouse husbandry and experimental procedures must be conducted in compliance with local, national, international legislation and guidelines on animal experimentation.
1. Induction of hair growth, burn induction and identification of long-term BrdU LRCs in the tail skin epithelium wholemounts
NOTE: Use 10-day or 7-week old C57BL/6 mice, preferably littermates, for the experimental designs described below. In all the experimental procedures, animals will be anesthetized by 3% isoflurane inhalation or euthanized by cervical dislocation as indicated.
- Induction of hair growth in the back skin of mice in the second telogen (resting) phase (about day 50 post-natal)
- Anesthetize mice with inhalation of 3% isoflurane. Confirm full deep anaesthesia by lack of a pedal reflex (firm toe pinch). Shave two independent side by side regions of the back skin in each single mouse using hair clippers and depilatory cream (Table of Materials). Use the left side for the control and the right side for treatment.
NOTE: Check that, after shaving, the subjacent back skin is pinkish and not grey/black, an indicator of melanogenesis and entrance into the anagen (growing) phase in this mouse strain. - Wash thoroughly with PBS to remove all cream remains and proceed to induction of transient in situ production of nonlethal ROS levels as described in section 2.1.
- Record hair follicle growth through daily acquisition of high-resolution images of the control and treated back skin areas in each animal (e.g., using an HD camera coupled to a 5−20x binocular lens).
- Anesthetize mice with inhalation of 3% isoflurane. Confirm full deep anaesthesia by lack of a pedal reflex (firm toe pinch). Shave two independent side by side regions of the back skin in each single mouse using hair clippers and depilatory cream (Table of Materials). Use the left side for the control and the right side for treatment.
- Induction of 2nd degree burn lesions in the back skin of mice in the second telogen (resting) phase (about day 50 post-natal)
- Anesthetize mice. Shave the whole back skin region in each single mouse using hair clippers and depilatory cream and wash thoroughly with PBS to remove all cream remains.
NOTE: Administer in situ subcutaneous injections of lidocaine 0.5% (2 mg/ml) in sterile saline, not exceeding 7 mg/ml, just before the burning procedure. - Preheat a brass bar (1 cm in cross-section) at 95 °C by immersing in boiling water and then apply on the central region of the dorsal back skin surface of each mouse for 5 s.
- Just after burn generation, intraperitoneally inject the animals with 1 mL of physiological solution (0.9% NaCl) on an electric blanket to prevent dehydration. Let the animals recover for 24 h and proceed to induction of transient in situ production of nonlethal ROS levels as described in section 2.3.
- Record burn wound progression through daily acquisition of high-resolution images of the control and treated back skin areas in each animal (e.g., using an HD camera coupled to a 5−20x binocular lens).
- Anesthetize mice. Shave the whole back skin region in each single mouse using hair clippers and depilatory cream and wash thoroughly with PBS to remove all cream remains.
- Generation and identification of long-term BrdU LRCs in the tail skin epithelium
- Inject 10/14-day old littermates intraperitoneally (no anaesthesia) once a day for 4 consecutive days with 50 mg/kg bodyweight BrdU dissolved in PBS. After the labelling phase, allow mice to grow for 50−60 days before any treatment.
NOTE: Use a fresh needle for each injection. - Proceed as described in section 2.3 for the induction of transient in situ nonlethal ROS production in the tail skin at different times before the preparation of tissue wholemounts.
- To prepare wholemounts of tail epidermis, euthanize mice by cervical dislocation and clip the tails with surgical scissors.
- Use a scalpel to make a straight longitudinal incision all along the tail and peel the whole skin as a single piece from the backbone. Incubate the peeled skin in 5 mM EDTA in PBS in 5 mL tubes for 4 h at 37 °C and carefully separate intact sheets of epidermis from the dermis using forceps.
- Fix the tissue in 4% formaldehyde in PBS for at least 72 h at room temperature (RT) and proceed for BrdU detection using appropriate antibodies.
- Use fluorescence/confocal microscopy to identify and quantify LRCs in each experimental condition, including light controls and photodynamic treatments at different times before the preparation of tissue wholemounts, as previously described in detail19,20.
NOTE: Fixed epidermal sheets may be stored in PBS containing 0.02% sodium azide at 4 °C for up to three months. Fixed epidermal sheets may be used for immunolocalization of required proteins following standard histological sections procedures.
- Inject 10/14-day old littermates intraperitoneally (no anaesthesia) once a day for 4 consecutive days with 50 mg/kg bodyweight BrdU dissolved in PBS. After the labelling phase, allow mice to grow for 50−60 days before any treatment.
2. Induction of transient production of nonlethal ROS levels in mouse skin
NOTE: To induce transient production of nonlethal ROS levels in mouse skin, a photodynamic treatment using a precursor of the endogenous photosensitizer PpIX, in this case, methyl-aminolevulinate (mALA), and red light will be used.
- To switch on transient ROS production for the induction of hair growth in the back skin, prepare the animals as indicated in section 1.1.
- Apply ~25 mg of mALA in the form of topical cream (Table of Materials) on the right region, keeping the left side as an internal control avoiding inter-individual differences. Incubate for 2.5 h in the dark, wash off the excess cream thoroughly with PBS. To confirm anesthesia depth, monitor the animals every 10 mins until fully recovered.
NOTE: The production of PpIX in the back skin should be tested in situ by its red fluorescence under blue light (407 nm) excitation. - Anesthetize the animals.
- Irradiate the whole back skin with an adequate red-light source (Table of Materials) for a total dose of 2.5−4 J/cm2. Keep mice on an electric blanket until their complete recovery and proceed as described in step 1.1.3.
NOTE: Irradiance should be adjusted by manipulating the distance between the light source and the tissue and measured using a power energy meter (Table of Materials). The experiment is considered finished when full hair growth is observed in any of the independent shaved regions of each animal. The whole procedure involves just a single photo treatment.
- Apply ~25 mg of mALA in the form of topical cream (Table of Materials) on the right region, keeping the left side as an internal control avoiding inter-individual differences. Incubate for 2.5 h in the dark, wash off the excess cream thoroughly with PBS. To confirm anesthesia depth, monitor the animals every 10 mins until fully recovered.
- To switch on transient ROS production for healing improvement of 2nd degree burn lesions, prepare the animals as indicated in section 1.2.
- Apply ~25 mg of mALA in the form of topical cream all along the burned surface, encompassing about 4 mm of adjacent tissue. Incubate for 2.5 h in the dark, wash off the excess cream thoroughly with PBS. To confirm anesthesia depth, monitor the animals every 10 mins until fully recovered.
- Anesthetize the animals.
- Irradiate the whole back skin with an adequate red-light source for a total dose of 2.5−4 J/cm2. Keep the mice on an electric blanket until their complete recovery and proceed as described in step 1.2.4.
NOTE: The experimental procedure for each animal is considered finished when full burn healing is observed. The whole procedure involves just a single photo treatment.
- To switch on transient ROS production in tail skin, prepare mice as indicated in section 1.3, apply ~25 mg of mALA in the form of topical cream all along the dorsal tissue area and proceed as described in section 2.1 for back skin. Perform photo treatments and correspondent light controls 24 h, 48 h, or 72 h before animal euthanasia and further extraction of tail skin whole mounts.
NOTE: In all experimental designs, assay the ROS-dependence of the process by using antioxidant ROS scavengers (e.g., daily inoculation of 100 mg/kg bodyweight N-acetyl-cysteine by intraperitoneal injection of a 20 mg/mL solution in PBS, pH 7.2, starting 5 days before mALA treatments or, alternatively, two doses of 100 mg/mL ascorbic acid in 50% ethanol, spaced 30 min, topically applied on the skin in the time interval between the mALA treatments and red-light irradiation).
3. ROS detection in the skin
- Ex vivo evaluation of ROS production in tail skin after photodynamic treatment using hydroethidine
NOTE: Hydroethidine is a non-fluorescent molecule that reacts specifically with ROS to produce fluorescent dye 2-hydroxyethidium (hET).- Incubate whole tail skins, obtained as described in section 1.3.3, for 3 h at 37 °C in 5 mM EDTA in PBS solution (control samples) or additionally containing 2 mM mALA (photodynamic treatment samples).
- In all cases, add hydroethidine to a final concentration of 3.2 μM from a 25 mg/mL stock in dimethyl sulfoxide (DMSO) and incubate for 1 h in the dark at RT.
- Stretch the tail skin samples over a glass surface using fingertips and irradiate with 636 nm red light at a 10 J/cm2 fluence.
- Proceed immediately to separate the epidermis from the dermis and to fix the epidermal sheets as described in section 1.3.3.
- Evaluate the hET red emission under a fluorescence/confocal microscope using green exciting light, capture high quality images and proceed with further analysis.
NOTE: Use hET staining of tissue samples in the absence of photodynamic treatment as a negative control for hET autoxidation.
- In vivo detection of ROS production in the back skin after induction of hair growth and burn healing followed by photodynamic treatment
NOTE: This step is performed using 2′,7′-dichlorodihydrofluorescein diacetate (DHF-DA), a cell permeant non-fluorescent compound that, after cleavage by intracellular esterase enzymes, specifically reacts with ROS giving the 2′,7′-dichlorofluorescein (DCF) fluorescent dye.- Use animals prepared for induction of hair growth (section 1.1) or burn healing (section 1.2). Just before topical mALA cream treatments (see sections 2.1 and 2.2), topically dispense 100 μL of 1 mg/mL in 50% ethanol of DHF-DA on all target control/treated skin areas, let the skin fully absorb the material and proceed forward with topical mALA cream application.
- Incubate treated animals for 4 h in the dark, wash the topical mALA cream thoroughly off the skin with PBS and dispense a second dose of 100 μL of DHF-DA solution on the skin. To confirm anesthesia depth, monitor the animals every 10 mins until fully recovered.
- Incubate treated animals for 50 min in the dark, anesthetize and irradiate the whole back skin with a fluence ranging from 2.5 to 4 J/cm2 of 636 nm red light using a LED lamp.
- Immediately after irradiation, evaluate ROS levels generated in the skin using an in vivo imaging system (Table of Materials). Set the filter box for 445−490 nm excitation and 515−575 nm emission, capture high quality images and proceed with further analysis.
NOTE: Use DHF-DA staining of tissue samples in the absence of photodynamic treatment as negative control for DHF-DA autoxidation.
Representative Results
The topical administration of the mALA precursor in the mouse back and tail skin results in a significant accumulation of PpIX in the whole tissue and, noticeably, in the hair follicle, as demonstrated by the reddish-pink fluorescence of this compound under blue light (407 nm) excitation (Figure 2A,C). Subsequent irradiation of treated tissue with red light (636 nm) at a fluence of 2.5−4 J/cm2 promotes transient production of ROS in the tissue, particularly in the bulge region of the hair follicle (Figure 2B,D).
Switching on nonlethal ROS production in mouse skin in vivo promotes a significant increase in the number of LRCs, categorized as somatic stem cells, in the bulge region of the hair follicle two days after photo treatments (Figure 3, left panels). Notably, the increase in the number of LRCs is transient, restoring to normal levels 6 days after treatments (Figure 3, right panel). As this region is one of the main stem cell niches in mouse skin, a transient induction of cell proliferation in this region mainly reflects the functional activation of the bulge niche and of the resident stem cell programs of proliferation and differentiation22,23.
The ROS-dependent activation of the bulge hair follicle niche is further associated with physiological responses in the skin. Thus, transient ROS production notably accelerates the skin healing process after a 2nd degree burn (Figure 4A,B). Quantification of the gradual reduction of the damaged/scab skin area demonstrates the robustness and statistical significance of the wound healing acceleration process induced by PpIX-dependent transient ROS production in the tissue (Figure 4C). In the same way, nonlethal ROS levels strongly promote hair growth after shaving during the second coordinated telogen (Figure 5A), a phase during which the hair follicle is refractory to respond to growth stimuli22,23, constituting an adequate way to evaluate the potential of new compounds and/or processes to stimulate hair growth. Notably, the use of antioxidant compounds like ascorbic acid (AA) results in a statistically significant reduction in the number of animals showing accelerated hair growth (Figure 5B). In addition, ROS production in the skin after photo treatments, quantified by the fluorescent emission of DHF in the skin, is also significantly reduced by antioxidant compounds (Figure 5C). Together, these results demonstrate that ROS production after PpIX-based photo treatments is strictly required to induce a physiological response in the tissue.
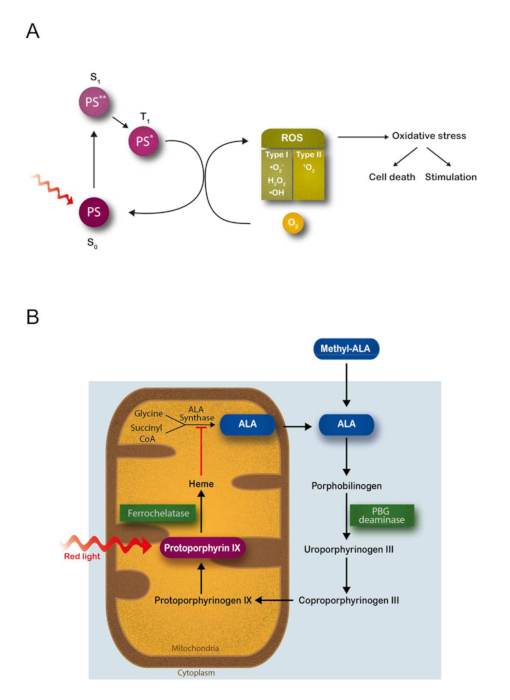
Figure 1: Theoretical background for the controlled switching on of endogenous photodynamic production of ROS in situ in cells and tissues using the heme biosynthetic pathway. (A) Schematic representation of the basic photochemical reactions resulting in molecular oxygen excitation during photodynamic treatments. Upon absorption of light with the appropriate λ, a photosensitizer molecule (PS) in the ground state S0 undergoes a transition to an excited singlet state S1. Since any excited state is energetically less preferable than the ground state, the molecule returns to S0 after a short period of time. Most PS have a high quantum efficiency for the transition from S1 to the triplet state T1, generally characterized by a relative long lifetime. Activated PS in the excited triplet state can react with other molecules via two different pathways. A type I photochemical reaction is the transfer of electrons to adjacent molecules to form radical species; these radicals are likely to react with molecular oxygen to produce ROS, including superoxide anion (•O2–), hydrogen peroxide (H2O2) and hydroxyl radical (•OH). A type II photochemical reaction represents the dominant process for most PS employed in PDT. During this reaction, the transfer of energy (not electrons) to molecular oxygen (whose configuration in the ground state is the triplet, 3O2) drives the formation of the non-radical but highly reactive singlet oxygen (1O2). The photoproducts formed during these reactions trigger a cascade of biochemical events resulting in an oxidative stress that finally causes cell death or that can potentially stimulate cell growth. (B) 5-aminolevulinic acid (ALA) is a natural precursor in the heme biosynthetic pathway, which involves both mitochondrial and cytosolic cellular compartments. The ALA synthase enzyme activity is regulated by a negative feedback control whereby free heme, the final product of this pathway, inhibits the synthesis of ALA from glycine and succinyl CoA. The administration of exogenous ALA or its derivative methyl aminolevulinate (mALA) bypasses the regulatory feedback system, so that downstream metabolites, especially protoporphyrin IX (PpIX), are accumulated in the cell inducing photosensitization. The rate-limiting characteristics of ferrochelatase, catalyst enzyme of the iron insertion in PpIX, promote the accumulation of this endogenous PS compound. PBG = porphobilinogen. This figure has been modified from Carrasco et al.19. Please click here to view a larger version of this figure.
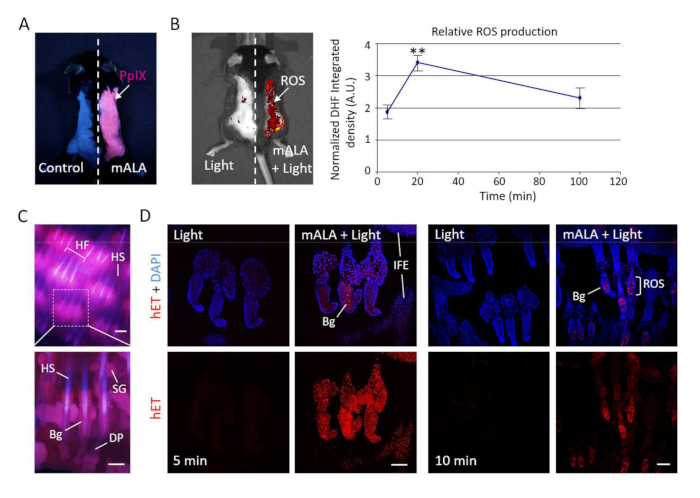
Figure 2: Photodynamic treatment with mALA and red light induces transient production of ROS in the skin. (A) Accumulation of endogenous PpIX after mALA topic treatment in back skin. The left side in the same animal was used as control. (B) Left panel: PpIX-dependent ROS (mALA+Light) production monitored by DHF-DA. Right panel: time-course analysis of relative ROS production in back skin; the relative integrated density of DHF-DA fluorescent emission of mALA+Light versus Light regions in each animal was quantified at different times after irradiation and normalized as described in methodology. The mean ± SE was represented (n = 4 for each time point). (C) Localization of PpIX in tail skin (fluorescence microscopy images). (D) ROS production in tail skin after mALA+Light as revealed by hET showing an increased and sustained accumulation in the bulge region of the hair follicle. Representative confocal microscopy images (maximum projections) are shown. Scale bar = 100 µm. This figure has been modified from Carrasco et al.19. Please click here to view a larger version of this figure.
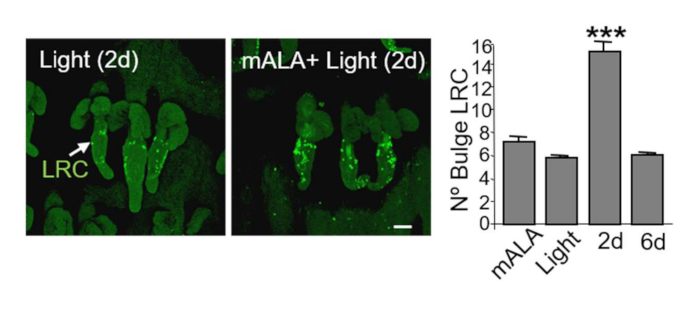
Figure 3: Switching on in situ ROS production in the skin promotes a significant increase of stem cells in the bulge region of the hair follicle niche. Left panels: representative confocal microscopy images (maximum projections) showing the localization of BrdU label retaining cells (LRC) in mouse tail skin whole mounts and the evident increase of LRC in the bulge region of hair follicles 2 days after PpIX-based phototreatments. Right panel: quantification of the number of LRC in the hair follicle bulge region. The mean + SE (n = 4) is represented. Scale bar = 50 µm. This figure has been modified from Carrasco et al.19. Please click here to view a larger version of this figure.
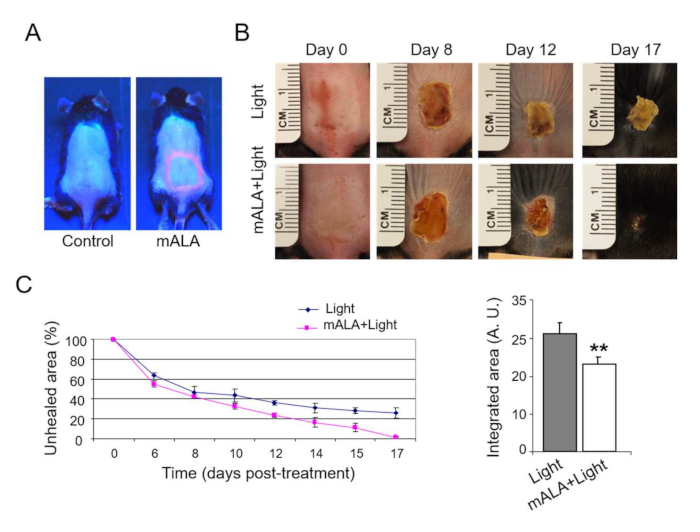
Figure 4: Switching on in situ ROS production in the skin accelerates burn healing. (A) PpIX production induced by mALA in burn injured regions in treated animals as compared to control samples. (B) Burn healing evolution in mALA+Light treated and control animals. (C) Time-course quantification of burned areas (left panel) showing accelerated burn healing in mALA+Light treated animals; the mean + SE (n = 4) of unhealed area is represented. Area-under-the-curve analysis (right panel) demonstrating statistical differences between both time-course curves (p ≤ 0.06). This figure has been modified from Carrasco et al.19. Please click here to view a larger version of this figure.
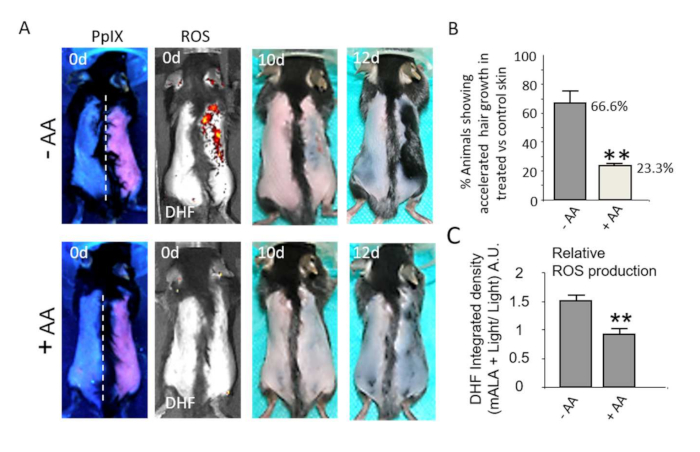
Figure 5: Switching on in situ ROS production in the skin stimulates hair growth. (A) Top row: Induction of hair growth during the refractory telogen phase by mALA+Light (right side of dorsal skin) as compared to light control region (left side). Bottom row: Both ROS production in the skin and the acceleration of hair growth induced by mALA+Light are inhibited by ascorbic acid (AA) antioxidant treatment. (B) Quantification of the % of animals showing accelerated hair growth in mALA-PT as compared to control region in the absence or presence of the antioxidant AA (n = 4 in 3 independent experiments). (C) Quantification of the ROS production inhibition in dorsal skin induced by AA during mALA-PT (n = 4). In all cases, bars represent mean + SE. This figure has been modified from Carrasco et al.19. Please click here to view a larger version of this figure.
Discussion
Here, we present a methodology that allows a transient activation of endogenous ROS production in vivo in mouse skin with physiological effects. The methodology is based on a photodynamic procedure to induce a controlled and local stimulation of the endogenous photosensitizer PpIX (Figure 1B). This experimental approach is an interesting tool to study ROS biology in in vivo experimental systems constituting a significant advance over methodologies using external ROS sources (usually hydrogen peroxide) and allowing controlled and local production of ROS in the tissue/sample.
Given that aminolevulinate-based precursors are administered in excess to promote the accumulation of PpIX inside the cells, a critical step in this methodology is the establishment of an adequate light dose to induce transient production of ROS levels in the tissue below the damage threshold but showing a strong stimulatory effect. Currently there are no available technologies to directly quantify the exact amount of any type of ROS that is produced in cells and tissues. In our methodology, it is still not possible to establish a direct correlation between a given light dose, the exact amount of ROS produced, and a given biological effect (e.g., cell death or cell proliferation). For this reason, the light dose (fluence) for any particular experimental model should be established empirically by the researcher using qualitative or semiqualitative parameters of choice for each situation. In the case of mouse skin, we choose an easily measurable transition between cell death and tissue damage and the induction of a significant and transient proliferative wave.
The methodology presented here has proven to be very effective in the improvement of skin regeneration in different processes, including burn healing and hair follicle growth. These observations pave the way for the implementation of therapeutic applications of this technology in clinics for the treatment of incidental or chronic burns and wounds or for different pathologies skin and, particularly, of the hair follicle involving a defective stem cell functioning.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work has been supported by grants from Ministerio de Economía y Competitividad (RTC-2014-2626-1 to JE) and Instituto de Salud Carlos III (PI15/01458 to JE) of Spain. EC has been supported by the Atracción de Talento Investigador grant 2017-T2/BMD-5766 (Comunidad de Madrid and UAM).
Materials
| 2′,7′-Dichlorofluorescin diacetate | Sigma Aldrich | D6883-50MG | |
| 5'-bromo-2'-deoxiuridine | Sigma Aldrich | B5002-500MG | |
| Anti-Bromodeoxyuridine-Fluorescein | Roche | 11202693001 | |
| Depilatory cream (e.g., Veet) | Veet | ||
| Dihydroethidium | Sigma Aldrich | 37291-25MG | |
| In Vivo imaging system, e.g., IVIS Lumina 2 | Perkin Elmer | ||
| mALA in the form of topical cream, e.g.,METVIX Crema 160 mg/g | Galderma | ||
| Power energy meter (e.g., ThorLabs Model PM100D) | ThorLabs | ||
| Red light source, e.g., 636 nm Aktilite LED lamp | Photocure ASA |
References
- Blázquez-Castro, A. Direct 1O2 optical excitation: A tool for redox biology. Redox Biology. 13, 39-59 (2017).
- Valko, M., et al. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology. 39 (1), 44-84 (2007).
- Sena, L. A., Chandel, N. S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Molecular Cell. 48 (2), 158-167 (2012).
- Bartosz, G. Reactive oxygen species: Destroyers or messengers. Biochemical Pharmacology. 77 (8), 1303-1315 (2009).
- Brieger, K., Schiavone, S., Miller, J., Krause, K. Reactive oxygen species: from health to disease. Swiss Medical Weekly. 142, 13659 (2012).
- Speakman, J. R., Selman, C. The free-radical damage theory: Accumulating evidence against a simple link of oxidative stress to ageing and lifespan. BioEssays. 33 (4), 255-259 (2011).
- Fernandez, V., Videla, L. A. Biochemical aspects of cellular antioxidant systems. Biological Research. 29 (2), 177-182 (1996).
- Matés, J. M., Sánchez-Jiménez, F. Antioxidant enzymes and their implications in pathophysiologic processes. Frontiers in Bioscience. 4, 339-345 (1999).
- Bedard, K., Krause, K. -. H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiological Reviews. 87 (1), 245-313 (2007).
- Leto, T. L., Morand, S., Hurt, D., Ueyama, T. Targeting and Regulation of Reactive Oxygen Species Generation by Nox Family NADPH Oxidases. Antioxidants & Redox Signaling. 11 (10), 2607-2619 (2009).
- Hernández-García, D., Wood, C. D., Castro-Obregón, S., Covarrubias, L. Reactive oxygen species: A radical role in development. Free Radical Biology and Medicine. 49 (2), 130-143 (2010).
- Covarrubias, L., Hernández-García, D., Schnabel, D., Salas-Vidal, E., Castro-Obregón, S. Function of reactive oxygen species during animal development: Passive or active. Developmental Biology. 320 (1), 1-11 (2008).
- Timme-Laragy, A. R., Hahn, M. E., Hansen, J. M., Rastogi, A., Roy, M. A. Redox stress and signaling during vertebrate embryonic development: Regulation and responses. Seminars in Cell & Developmental Biology. 80, 17-28 (2018).
- Owusu-Ansah, E., Banerjee, U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 461 (7263), 537-541 (2009).
- Love, N. R., et al. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nature Cell Biology. 15 (2), 222-228 (2013).
- Le Belle, J. E., et al. Proliferative Neural Stem Cells Have High Endogenous ROS Levels that Regulate Self-Renewal and Neurogenesis in a PI3K/Akt-Dependant Manner. Cell Stem Cell. 8 (1), 59-71 (2011).
- Myant, K. B., et al. production and NF-κB activation triggered by RAC1 facilitate WNT-driven intestinal stem cell proliferation and colorectal cancer initiation. Cell Stem Cell. 12 (6), 761-773 (2013).
- Hamanaka, R. B., et al. Mitochondrial Reactive Oxygen Species Promote Epidermal Differentiation and Hair Follicle Development. Science Signaling. 6 (261), 8 (2013).
- Carrasco, E., et al. Photoactivation of ROS Production in situ Transiently Activates Cell Proliferation in Mouse Skin and in the hair Follicle Stem Cell Niche Promoting Hair Growth and Wound Healing. Journal of Investigative Dermatology. 135 (11), 1-12 (2015).
- Carrasco, E., Blázquez-Castro, A., Calvo, M. I., Juarranz, &. #. 1. 9. 3. ;., Espada, J. Switching on a transient endogenous ROS production in mammalian cells and tissues. Methods. , 109 (2016).
- Braun, K. M., et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 130 (21), 5241-5255 (2003).
- Hsu, Y. -. C., Li, L., Fuchs, E. Emerging interactions between skin stem cells and their niches. Nature Medicine. 20 (8), 847-856 (2014).
- Plikus, M. V., et al. Epithelial stem cells and implications for wound repair. Seminars in Cell & Developmental Biology. 23 (9), 946-953 (2012).

